Lateral Bias in Visual Working Memory
Abstract
:Highlights
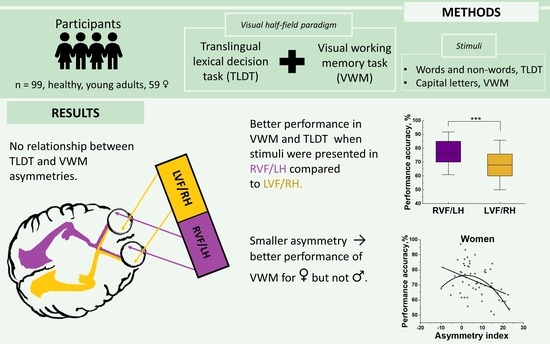
- Greater performance in men and women when letters presented in RVF.
- Smaller asymmetry related to better performance for women but not men.
- Lateral bias in visual working memory was independent from lateralization of lexical decisions.
Abstract
1. Introduction
1.1. Visual Change Detection Task: Classical Version and Bilateral Modification
1.2. Stimuli in Change Detection VWM Studies
1.3. Asymmetry–Performance Relationships
1.4. Aims and Hypotheses of the Present Study
2. Methods
2.1. Participants
2.2. Demographic Questionnaire
2.3. Handedness
2.4. Language Lateralization
2.5. VWM Task
2.6. General Procedure
2.7. Data Analysis
3. Results
3.1. VWM Performance Accuracy
3.2. VWM Response Time
3.3. VWM Capacity
3.4. VWM Asymmetry Indices
3.5. TLDT Performance
3.6. Relationship between TLDT and VWM Performance
3.7. VWM Performance Comparison between Participants with Typical and Atypical TLDT Asymmetry
3.8. Asymmetry–Performance Relationships in VWM
4. Discussion
4.1. Asymmetry–Performance Relationships
4.2. Sex Effect
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nyberg, L.; Eriksson, J. Working Memory: Maintenance, Updating, and the Realization of Intentions. Cold Spring Harb. Perspect. Biol. 2015, 8, a021816. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Vogel, E.; Mayr, U.; Awh, E. Quantity, Not Quality: The Relationship between Fluid Intelligence and Working Memory Capacity. Psychon. Bull. Rev. 2010, 17, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unsworth, N.; Fukuda, K.; Awh, E.; Vogel, E.K. Working Memory Delay Activity Predicts Individual Differences in Cognitive Abilities. J. Cogn. Neurosci. 2015, 27, 853–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healy, A.F.; Jr, L.E.B. Foreign Language Learning: Psycholinguistic Studies on Training and Retention; Psychology Press: London, UK, 2013; ISBN 978-1-134-80766-6. [Google Scholar]
- Luck, S.J.; Vogel, E.K. The Capacity of Visual Working Memory for Features and Conjunctions. Nature 1997, 390, 279–281. [Google Scholar] [CrossRef]
- Phillips, W.A. On the Distinction between Sensory Storage and Short-Term Visual Memory. Percept. Psychophys. 1974, 16, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Allon, A.S.; Vixman, G.; Luria, R. Gestalt Grouping Cues Can Improve Filtering Performance in Visual Working Memory. Psychol. Res. 2019, 83, 1656–1672. [Google Scholar] [CrossRef] [PubMed]
- Awh, E.; Barton, B.; Vogel, E.K. Visual Working Memory Represents a Fixed Number of Items Regardless of Complexity. Psychol. Sci. 2007, 18, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, Y.; Gan, S.; Du, F. The Reliability of Estimating Visual Working Memory Capacity. Sci. Rep. 2019, 9, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouder, J.N.; Morey, R.D.; Morey, C.C.; Cowan, N. How to Measure Working Memory Capacity in the Change Detection Paradigm. Psychon. Bull. Rev. 2011, 18, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Vogel, E.K.; Machizawa, M.G. Neural Activity Predicts Individual Differences in Visual Working Memory Capacity. Nature 2004, 428, 748–751. [Google Scholar] [CrossRef]
- Machizawa, M.G.; Driver, J. Principal Component Analysis of Behavioural Individual Differences Suggests That Particular Aspects of Visual Working Memory May Relate to Specific Aspects of Attention. Neuropsychologia 2011, 49, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.C.; Werkle-Bergner, M.; Lindenberger, U. Contralateral Delay Activity Reveals Life-Span Age Differences in Top-down Modulation of Working Memory Contents. Cereb. Cortex. 2011, 21, 2809–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Chun, M.M. Dissociable Neural Mechanisms Supporting Visual Short-Term Memory for Objects. Nature 2006, 440, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Clapp, W.; Kirk, I.J.; Hausmann, M. Effects of Memory Load on Hemispheric Asymmetries of Colour Memory. Laterality 2007, 12, 139–153. [Google Scholar] [CrossRef]
- Cutini, S.; Scarpa, F.; Scatturin, P.; Jolicœur, P.; Pluchino, P.; Zorzi, M.; Dell’Acqua, R. A Hemodynamic Correlate of Lateralized Visual Short-Term Memories. Neuropsychologia 2011, 49, 1611–1621. [Google Scholar] [CrossRef]
- Diamantopoulou, S.; Poom, L.; Klaver, P.; Talsma, D. Visual Working Memory Capacity and Stimulus Categories: A Behavioral and Electrophysiological Investigation. Exp. Brain Res. 2011, 209, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.M.; Federmeier, K.D. The Memory That’s Right and the Memory That’s Left: Event-Related Potentials Reveal Hemispheric Asymmetries in the Encoding and Retention of Verbal Information. Neuropsychologia 2007, 45, 1777–1790. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.M.; Federmeier, K.D. Left and Right Memory Revisited: Electrophysiological Investigations of Hemispheric Asymmetries at Retrieval. Neuropsychologia 2009, 47, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Machizawa, M.G.; Driver, J.; Watanabe, T. Gray Matter Volume in Different Cortical Structures Dissociably Relates to Individual Differences in Capacity and Precision of Visual Working Memory. Cereb. Cortex 2020, 30, 4759–4770. [Google Scholar] [CrossRef]
- McCollough, A.W.; Machizawa, M.G.; Vogel, E.K. Electrophysiological Measures of Maintaining Representations in Visual Working Memory. Cortex 2007, 43, 77–94. [Google Scholar] [CrossRef]
- Robitaille, N.; Grimault, S.; Jolicœur, P. Bilateral Parietal and Contralateral Responses during Maintenance of Unilaterally Encoded Objects in Visual Short-Term Memory: Evidence from Magnetoencephalography. Psychophysiology 2009, 46, 1090–1099. [Google Scholar] [CrossRef]
- Sheremata, S.L.; Bettencourt, K.C.; Somers, D.C. Hemispheric Asymmetry in Visuotopic Posterior Parietal Cortex Emerges with Visual Short-Term Memory Load. J. Neurosci. 2010, 30, 12581–12588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheremata, S.; Shomstein, S. Hemifield Asymmetries Differentiate VSTM for Single- and Multiple-Feature Objects. Atten. Percept. Psychophys. 2014, 76, 1609–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voytek, B.; Knight, R.T. Prefrontal Cortex and Basal Ganglia Contributions to Visual Working Memory. Proc. Natl. Acad. Sci. USA 2010, 107, 18167–18172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kail, R.V.; Siegel, A.W. Sex and Hemispheric Differences in the Recall of Verbal and Spatial Information. Cortex 1978, 14, 557–563. [Google Scholar] [CrossRef]
- Jonides, J.; Smith, E.E.; Koeppe, R.A.; Awh, E.; Minoshima, S.; Mintun, M.A. Spatial Working Memory in Humans as Revealed by PET. Nature 1993, 363, 623–625. [Google Scholar] [CrossRef] [Green Version]
- De Renzi, E.; Faglioni, P.; Previdi, P. Spatial Memory and Hemispheric Locus of Lesion. Cortex J. Devoted Study Nerv. Syst. Behav. 1977, 13, 424–433. [Google Scholar] [CrossRef]
- Rajsic, J.; Burton, J.A.; Woodman, G.F. Contralateral Delay Activity Tracks the Storage of Visually Presented Letters and Words. Psychophysiology 2019, 56, e13282. [Google Scholar] [CrossRef] [Green Version]
- Joseph, J.E.; Swearingen, J.; Corbly, C.R.; Curry, T.E.; Kelly, T.H. Influence of Estradiol on Functional Brain Organization for Working Memory. Neuroimage 2012, 59, 2923–2931. [Google Scholar] [CrossRef] [Green Version]
- Petrides, M.; Alivisatos, B.; Meyer, E.; Evans, A.C. Functional Activation of the Human Frontal Cortex during the Performance of Verbal Working Memory Tasks. Proc. Natl. Acad. Sci. USA 1993, 90, 878–882. [Google Scholar] [CrossRef]
- Sternberg, S. Memory-Scanning: Mental Processes Revealed by Reaction-Time Experiments. Am. Sci. 1969, 57, 421–457. [Google Scholar] [PubMed]
- Baddeley, A. Working Memory: Looking Back and Looking Forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef]
- Bishop, D.V.M. Cerebral Asymmetry and Language Development: Cause, Correlate, or Consequence? Science 2013, 340, 1230531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axmacher, N.; Bialleck, K.A.; Weber, B.; Helmstaedter, C.; Elger, C.E.; Fell, J. Working Memory Representation in Atypical Language Dominance. Hum. Brain Mapp. 2008, 30, 2032–2043. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, M.; Brysbaert, M.; van der Haegen, L.; Lewald, J.; Specht, K.; Hirnstein, M.; Willemin, J.; Barton, J.; Buchilly, D.; Chmetz, F.; et al. Language Lateralisation Measured across Linguistic and National Boundaries. Cortex 2019, 111, 134–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willemin, J.; Hausmann, M.; Brysbaert, M.; Dael, N.; Chmetz, F.; Fioravera, A.; Gieruc, K.; Mohr, C. Stability of Right Visual Field Advantage in an International Lateralized Lexical Decision Task Irrespective of Participants’ Sex, Handedness or Bilingualism. Laterality Asymmetries Body Brain Cogn. 2016, 21, 502–524. [Google Scholar] [CrossRef] [Green Version]
- Bourne, V.J. The Divided Visual Field Paradigm: Methodological Considerations. Laterality 2006, 11, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Krach, S.; Chen, L.M.; Hartje, W. Comparison between Visual Half-Field Performance and Cerebral Blood Flow Changes as Indicators of Language Dominance. Laterality 2006, 11, 122–140. [Google Scholar] [CrossRef]
- Esteves, M.; Lopes, S.S.; Almeida, A.; Sousa, N.; Leite-Almeida, H. Unmasking the Relevance of Hemispheric Asymmetries—Break on through (to the Other Side). Prog. Neurobiol. 2020, 192, 101823. [Google Scholar] [CrossRef] [PubMed]
- Güntürkün, O.; Ströckens, F.; Ocklenburg, S. Brain Lateralization: A Comparative Perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef]
- Rogers, L.J. Brain Lateralization and Cognitive Capacity. Animals 2021, 11, 1996. [Google Scholar] [CrossRef]
- Chiarello, C.; Welcome, S.E.; Halderman, L.K.; Leonard, C.M. Does Degree of Asymmetry Relate to Performance? An Investigation of Word Recognition and Reading in Consistent and Mixed Handers. Brain Cogn. 2009, 69, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everts, R.; Lidzba, K.; Wilke, M.; Kiefer, C.; Mordasini, M.; Schroth, G.; Perrig, W.; Steinlin, M. Strengthening of Laterality of Verbal and Visuospatial Functions during Childhood and Adolescence. Hum. Brain Mapp. 2009, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Gotts, S.J.; Jo, H.J.; Wallace, G.L.; Saad, Z.S.; Cox, R.W.; Martin, A. Two Distinct Forms of Functional Lateralization in the Human Brain. Proc. Natl. Acad. Sci. USA 2013, 110, E3435–E3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirnstein, M.; Hugdahl, K.; Hausmann, M. How Brain Asymmetry Relates to Performance–a Large-Scale Dichotic Listening Study. Front. Psychol. 2014, 4, 997. [Google Scholar] [CrossRef] [Green Version]
- Mellet, E.; Zago, L.; Jobard, G.; Crivello, F.; Petit, L.; Joliot, M.; Mazoyer, B.; Tzourio-Mazoyer, N. Weak Language Lateralization Affects Both Verbal and Spatial Skills: An FMRI Study in 297 Subjects. Neuropsychologia 2014, 65, 56–62. [Google Scholar] [CrossRef]
- Plessen, K.J.; Hugdahl, K.; Bansal, R.; Hao, X.; Peterson, B.S. Sex, Age, and Cognitive Correlates of Asymmetries in Thickness of the Cortical Mantle Across the Life Span. J. Neurosci. 2014, 34, 6294–6302. [Google Scholar] [CrossRef] [Green Version]
- Bartha-Doering, L.; Kollndorfer, K.; Kasprian, G.; Novak, A.; Schuler, A.-L.; Fischmeister, F.P.S.; Alexopoulos, J.; Gaillard, W.D.; Prayer, D.; Seidl, R.; et al. Weaker Semantic Language Lateralization Associated with Better Semantic Language Performance in Healthy Right-Handed Children. Brain Behav. 2018, 8, e01072. [Google Scholar] [CrossRef] [Green Version]
- Catani, M.; Allin, M.P.G.; Husain, M.; Pugliese, L.; Mesulam, M.M.; Murray, R.M.; Jones, D.K. Symmetries in Human Brain Language Pathways Correlate with Verbal Recall. Proc. Natl. Acad. Sci. USA 2007, 104, 17163–17168. [Google Scholar] [CrossRef] [Green Version]
- Hirnstein, M.; Leask, S.; Rose, J.; Hausmann, M. Disentangling the Relationship between Hemispheric Asymmetry and Cognitive Performance. Brain Cogn. 2010, 73, 119–127. [Google Scholar] [CrossRef]
- Làdavas, E.; Umiltà, C. Do Laterality Measures Relate to Speed of Response in Central Vision? Brain Cogn. 1983, 2, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Boles, D.B.; Barth, J.M.; Merrill, E.C. Asymmetry and Performance: Toward a Neurodevelopmental Theory. Brain Cogn. 2008, 66, 124–139. [Google Scholar] [CrossRef]
- Nagel, B.J.; Herting, M.M.; Maxwell, E.C.; Bruno, R.; Fair, D. Hemispheric Lateralization of Verbal and Spatial Working Memory during Adolescence. Brain Cogn. 2013, 82, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter-Lorenz, P.A.; Jonides, J.; Smith, E.E.; Hartley, A.; Miller, A.; Marshuetz, C.; Koeppe, R.A. Age Differences in the Frontal Lateralization of Verbal and Spatial Working Memory Revealed by PET. J. Cogn. Neurosci. 2000, 12, 174–187. [Google Scholar] [CrossRef]
- Boles, D.B. A Large-Sample Study of Sex Differences in Functional Cerebral Lateralization. J. Clin. Exp. Neuropsychol. 2005, 27, 759–768. [Google Scholar] [CrossRef]
- Leask, S.J.; Crow, T.J. Word Acquisition Reflects Lateralization of Hand Skill. Trends Cogn. Sci. 2001, 5, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Hausmann, M. Why Sex Hormones Matter for Neuroscience: A Very Short Review on Sex, Sex Hormones, and Functional Brain Asymmetries. J. Neurosci. Res. 2017, 95, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Forster, K.I.; Forster, J.C. DMDX: A Windows Display Program with Millisecond Accuracy. Behav. Res. Methods Instrum. Comput. 2003, 35, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keuleers, E.; Brysbaert, M. Wuggy: A Multilingual Pseudoword Generator. Behav. Res. Methods 2010, 42, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Sheremata, S.L.; Somers, D.C. Role of Encoding Duration on Visual-Short Term Memory Capacity. J. Vis. 2008, 8, 1173. [Google Scholar] [CrossRef]
- Bortz, J.; Gustav, A.; Lienert; Boehnke, K. Verteilungsfreie Methoden in der Biostatistik; Springer-Lehrbuch; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-74706-2. [Google Scholar]
- Beaumont, J.G. Divided Visual Field Studies of Cerebral Organization; Academic Press: London, UK; New York, NY, USA, 1982; ISBN 978-0-12-084080-9. [Google Scholar]
- Mesulam, M. Functional Anatomy of Attention and Neglect: From Neurons to Networks. In The Cognitive and Neural Bases of Spatial Neglect; Oxford University Press: Oxford, UK, 2002; ISBN 978-0-19-850833-5. [Google Scholar]
- Desimone, R.; Duncan, J. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, C.; Guo, J.; Kong, Y.; Li, H.; Du, B.; Ding, Y.; Song, Y. Visual Working Memory Guides Spatial Attention: Evidence from Alpha Oscillations and Sustained Potentials. Neuropsychologia 2021, 151, 107719. [Google Scholar] [CrossRef]
- Kermani, M.; Verghese, A.; Vidyasagar, T.R. Attentional Asymmetry between Visual Hemifields Is Related to Habitual Direction of Reading and Its Implications for Debate on Cause and Effects of Dyslexia. Dyslexia 2018, 24, 33–43. [Google Scholar] [CrossRef]
- Rima, S.; Kerbyson, G.; Jones, E.; Schmid, M.C. Advantage of Detecting Visual Events in the Right Hemifield Is Affected by Reading Skill. Vis. Res. 2020, 169, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hirnstein, M.; Hausmann, M.; Güntürkün, O. The Evolutionary Origins of Functional Cerebral Asymmetries in Humans: Does Lateralization Enhance Parallel Processing? Behav. Brain Res. 2008, 187, 297–303. [Google Scholar] [CrossRef]
- Jäncke, L.; Steinmetz, H. Interhemispheric Transfer Time and Corpus Callosum Size. Neuroreport 1994, 5, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Lust, J.M.; Geuze, R.H.; Groothuis, A.G.G.; Bouma, A. Functional Cerebral Lateralization and Dual-Task Efficiency—Testing the Function of Human Brain Lateralization Using FTCD. Behav. Brain Res. 2011, 217, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Menzler, K.; Belke, M.; Wehrmann, E.; Krakow, K.; Lengler, U.; Jansen, A.; Hamer, H.M.; Oertel, W.H.; Rosenow, F.; Knake, S. Men and Women Are Different: Diffusion Tensor Imaging Reveals Sexual Dimorphism in the Microstructure of the Thalamus, Corpus Callosum and Cingulum. NeuroImage 2011, 54, 2557–2562. [Google Scholar] [CrossRef]
- Draca, S. Gender-Specific Functional Cerebral Asymmetries and Unilateral Cerebral Lesion Sequelae. Rev. Neurosci. 2010, 21, 421–425. [Google Scholar] [CrossRef]
- Innes, B.R.; Burt, D.M.; Birch, Y.K.; Hausmann, M. A Leftward Bias However You Look at It: Revisiting the Emotional Chimeric Face Task as a Tool for Measuring Emotion Lateralization. Laterality 2016, 21, 643–661. [Google Scholar] [CrossRef] [Green Version]
- Nicholls, M.E.; Roberts, G.R. Can Free-Viewing Perceptual Asymmetries Be Explained by Scanning, Pre-Motor or Attentional Biases? Cortex 2002, 38, 113–136. [Google Scholar] [CrossRef] [PubMed]
- Smekal, V.; Burt, D.M.; Kentridge, R.W.; Hausmann, M. Emotion Lateralization in a Graduated Emotional Chimeric Face Task: An Online Study. Neuropsychology 2022, 36, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Hodgetts, S.; Hausmann, M. Sex/Gender Differences in Brain Lateralisation and Connectivity; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Bayer, U.; Kessler, N.; Güntürkün, O.; Hausmann, M. Interhemispheric Interaction during the Menstrual Cycle. Neuropsychologia 2008, 46, 2415–2422. [Google Scholar] [CrossRef]
- Bayer, U.; Erdmann, G. The Influence of Sex Hormones on Functional Cerebral Asymmetries in Postmenopausal Women. Brain Cogn. 2008, 67, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; De Tommaso, M.; Cianci, A.; Colacurci, N.; Rella, L.; Loiudice, L.; Cicinelli, M.V.; Livrea, P. Oral Contraceptive Therapy Modulates Hemispheric Asymmetry in Spatial Attention. Contraception 2011, 84, 634–636. [Google Scholar] [CrossRef]
- Hausmann, M. Hemispheric Asymmetry in Spatial Attention across the Menstrual Cycle. Neuropsychologia 2005, 43, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.; Hausmann, M.; Stoffers, B.; Vohn, R.; Kellermann, T.; Sturm, W. Estradiol Modulates Functional Brain Organization during the Menstrual Cycle: An Analysis of Interhemispheric Inhibition. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 13401–13410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouder, J.N.; Haaf, J.M. A Psychometrics of Individual Differences in Experimental Tasks. Psychon. Bull. Rev. 2019, 26, 452–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausmann, M.; Hodgetts, S.; Eerola, T. Music-Induced Changes in Functional Cerebral Asymmetries. Brain Cogn. 2016, 104, 58–71. [Google Scholar] [CrossRef] [PubMed]






| Accuracy r (Optimal AI) | Memory Capacity r (Optimal AI) | Response Time r (Optimal AI) | |
|---|---|---|---|
| Sided (directional)AIs | |||
| Linear regression | |||
| All participants | −0.29 ** | −0.17 # | 0.005 |
| Women | −0.40 ** | −0.26 # | 0.004 |
| Men | −0.13 | −0.06 | 0.027 |
| Quadratic regression | |||
| All participants | −0.41 (1.61) *** | −0.25 (1.82) * | −0.13 (−1.09) |
| Women | −0.51 (2.36) *** | −0.26 (−6.67) | −0.18 (−2.55) |
| Men | −0.29 (2.38) | −0.28 (4.88) | −0.07 (−1.09) |
| Absolute AIs | |||
| Linear regression | |||
| All participants | −0.39 *** | −0.25 ** | −0.10 |
| Women | −0.49 *** | −0.29 * | −0.12 |
| Men | −0.21 | −0.20 | −0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grikšienė, R.; Gaizauskaite, R.; Pretkelyte, I.; Hausmann, M. Lateral Bias in Visual Working Memory. Symmetry 2022, 14, 2509. https://0-doi-org.brum.beds.ac.uk/10.3390/sym14122509
Grikšienė R, Gaizauskaite R, Pretkelyte I, Hausmann M. Lateral Bias in Visual Working Memory. Symmetry. 2022; 14(12):2509. https://0-doi-org.brum.beds.ac.uk/10.3390/sym14122509
Chicago/Turabian StyleGrikšienė, Ramunė, Rimante Gaizauskaite, Indre Pretkelyte, and Markus Hausmann. 2022. "Lateral Bias in Visual Working Memory" Symmetry 14, no. 12: 2509. https://0-doi-org.brum.beds.ac.uk/10.3390/sym14122509