The Perron Gold Deposit, Archean Abitibi Belt, Canada: Exceptionally High-Grade Mineralization Related to Higher Gold-Carrying Capacity of Hydrocarbon-Rich Fluids
Abstract
:1. Introduction
2. Regional and Local Geology
3. Gold Mineralizations
3.1. Primary Sulfide-Rich Gold Mineralization
3.2. Orogenic Quartz-Vein Type
4. Material and Methods
5. Result
5.1. Sulfide Mineralogy and Gold Habit
5.2. Fluid Inclusion Petrography
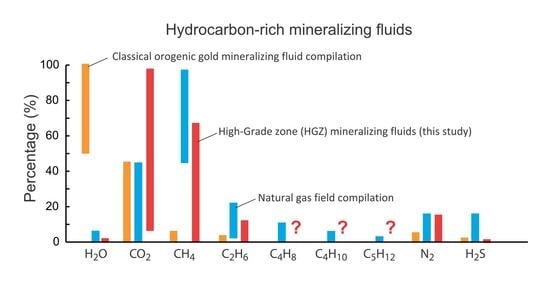
5.3. Volatile Composition of Fluids in the HGZ
5.4. Pyrite Composition
5.5. Sphalerite GGIMFis Geothermometer
5.6. Amphibole Thermobarometry
6. Discussion
6.1. Tectono-Thermal Evolution
6.2. Gold Precipitating Mechanisms and Remobilization
6.3. Gold Solubility and Fluid Source
6.4. Potential Source of Gold
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontboté, L.; Kouzmanov, K.; Chiaradia, M.; Pokrovski, G.S. Sulfide Minerals in Hydrothermal Deposits. Elements 2017, 13, 97–103. [Google Scholar] [CrossRef]
- Dubé, B.; Williamson, K.; McNicoll, V.; Malo, M.; Skulski, T.; Twomey, T.; Sanborn-Barrie, M. Timing of gold mineralization at Red Lake, northwestern Ontario, Canada: New constrainsts from U-Pb geochronology at the Goldcorp High-Grade zone, Red Lake mine, and the Madsen mine. Econ. Geol. 2004, 99, 1611–1641. [Google Scholar] [CrossRef]
- Card, K.D. A review of the Superior Province of the Canadian Shield, a product of Archean accretion. Precambrian Res. 1990, 48, 99–156. [Google Scholar] [CrossRef]
- Chown, E.H.; Daigneault, R.; Mueller, W. Tectonic evolution of the Northern Volcanic Zone, Abitibi belt, Quebec. Can. J. Earth Sci. 1992, 29, 2211–2225. [Google Scholar] [CrossRef]
- Gaboury, D. Geochemical approaches in the discrimination of synvolcanic intrusions as a guide for volcanogenic base metal exploration: Example from the Abitibi belt, Canada. Appl. Earth Sci. 2006, 115, 71–79. [Google Scholar] [CrossRef]
- Robert, F.; Poulsen, K.H. World-class Archaean gold deposits in Canada: An overview. Aust. J. Earth Sci. 1997, 44, 329–351. [Google Scholar] [CrossRef]
- Dubé, B.; Mercier-Langevin, P.; Ayer, J.; Pilote, J.-L.; Monecke, T. Gold Deposits of the World-Class Timmins-Porcupine Camp, Abitibi Greenstone Belt, Canada; Special Publication; Society of Economic Geologists: Littleton, CO, USA, 2020; Volume 23, pp. 53–80. [Google Scholar]
- Rodney, A.L.; Weihed, P. Global comparisons of volcanic-associated massive sulphide districts. Geol. Soc. Spec. Publ. 2002, 204, 13–37. [Google Scholar]
- Gaboury, D.; Pearson, V. Rhyolite Geochemical Signatures and Association with Volcanogenic Massive Sulfide Deposits: Examples from the Abitibi Belt, Canada. Econ. Geol. 2008, 103, 1531–1562. [Google Scholar] [CrossRef]
- Lafrance, B.; Mueller, W.U.; Daigneault, R.; Dupras, N. Evolution of a submerged composite arc volcano: Volcanology and geochemistry of the Normétal volcanic complex, Abitibi greenstone belt, Québec, Canada. Precambrian Res. 2000, 101, 277–311. [Google Scholar] [CrossRef]
- Mortensen, J.K. U-Pb gechronology of the eastern Abitibi Subprovince. Part 1: Chibougamau-Matagami-Joutel. Can. J. Earth Sci. 1993, 30, 11–28. [Google Scholar] [CrossRef]
- Barrett, T.J.; Ayer, J.A.; Ordóñez-Calderón, J.C.; Hamilton, M.A. Burntbush-Normétal volcanic belt, Abitibi greenstone belt, Ontario-Quebec: Geological mapping and compilation project, Discover Abitibi Initiative. In Geological Mapping and Compilation of the Burntbush-Normétal Volcanic Belt, Abitibi Greenstone Belt, Ontario-Quebec, Miscellaneous Release—Data 299; Ontario Geological Survey: Sudbury, ON, Canada, 2013; 135p. [Google Scholar]
- Teasdale, N. Regional Study of Geochemical Alteration Associated with the Normétal Deposit Abitibi Greenstone Belt, Québec. Master’s Thesis, École Polytechnique, Montreal, QC, Canada, 1993; 160p. [Google Scholar]
- Lesher, C.M.; Goodwin, A.M.; Campbell, I.H.; Gorton, M.P. Trace-element geochemistry of ore-associated and barren, felsic metavolcanic rocks in the Superior Province, Canada. Can. J. Earth Sci. 1986, 23, 222–237. [Google Scholar] [CrossRef]
- Zhang, Q.; Machado, N.; Ludden, J.N.; Moore, D. Geotectonic Constraints from U-Pb Ages for the Blake River Group, the Kinojevis Group and the Normetal Mine Area, Abitibi, Quebec. Program and Abstract; MAC/AMC: Edmonton, AB, Canada, 1993; p. A-114. [Google Scholar]
- Lafrance, B. Reconstruction d’un Environnement de Sulfures Massifs Volcanogènes Déformés: Exemple Archéen de Normétal, Abitibi. Unpublished. Ph.D. Thesis, Université du Québec à Chicoutimi, Chicoutimi, QC, Canada, 2003; 362p. [Google Scholar]
- Robert, F.; Poulsen, K.H. Vein Formation and deformation in greenstone gold deposits. Rev. Econ. Geol. 2001, 13, 111–155. [Google Scholar]
- Gaboury, D.; Keita, M.; Guha, J.; Lu, H.-Z. Mass spectrometric analysis of volatiles in fluid inclusions decrepitated by controlled heating under vacuum. Econ. Geol. 2008, 103, 439–443. [Google Scholar] [CrossRef]
- Genna, D.; Gaboury, D. Deciphering the Hydrothermal Evolution of a VMS System by LA-ICP-MS Using Trace Elements in Pyrite: An Example from the Bracemac-McLeod Deposits, Abitibi, Canada, and Implications for Exploration. Econ. Geol. 2015, 110, 2087–2108. [Google Scholar] [CrossRef]
- Augustin, J.; Gaboury, D. Multi-stage and multi-sourced fluid and gold in the formation of orogenic gold deposits in the world-class Mana district of Burkina Faso-Revealed by LA-ICP-MS analysis of pyrites and arsenopyrites. Ore Geol. Rev. 2019, 104, 495–521. [Google Scholar] [CrossRef]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Locock, A.J. An Excel spreadsheet to classify chemical analyses of amphiboles following the IMA 2012 recommendations. Comput. Geosci. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- Zenk, M.; Schulz, B. Zoned Ca-amphiboles and related P-T evolution in metabasites from the classical Barrovian metamorphic zones in Scotland. Miner. Mag. 2004, 68, 769–786. [Google Scholar] [CrossRef]
- Boullier, A.-M.; Robert, F. Palaeoseismic events recorded in Archaean gold-quartz vein networks, Val d’Or, Abitibi, Quebec, Canada. J. Struct. Geol. 1992, 14, 161–179. [Google Scholar] [CrossRef]
- Ridley, J.R.; Diamond, L.W. Fluid chemistry of orogenic lode gold deposits and implications for genetic models. Rev. Econ. Geol. 2000, 13, 146–162. [Google Scholar]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Lecumberri-Sanchez, P.; Moncada, D.; Steele-MacInnis, M. Fluid Inclusions in Hydrothermal Ore Deposits. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; Volume 13, pp. 119–142. [Google Scholar]
- Li, X.-H.; Klyukin, Y.I.; Steele-MacInnis, M.; Fan, H.-R.; Yang, K.-F.; Zoheir, B. Phase equilibria, thermodynamic properties, and solubility of quartz in salineaqueous-carbonic fluids: Application to orogenic and intrusion-related gold deposits. Geochim. Cosmochim. Acta 2020, 283, 201–221. [Google Scholar] [CrossRef]
- Tuba, G.; Kontak, D.J.; Choquette, B.G.; Pfister, J.; Hastie, E.C.G.; van Hees, E.H.P. Fluid diversity in the gold-endowed Archean orogenic systems of the Abitibi greenstone belt (Canada) I: Constraining the PTX of prolonged hydrothermal systems. Ore Geol. Rev. 2021, 135, 104221. [Google Scholar] [CrossRef]
- Yardley, B.W.D.; Bodnar, R.J. Fluids in the continental crust. Geochem. Perspect. 2014, 3, 123. [Google Scholar] [CrossRef] [Green Version]
- Prokofiev, V.Y.; Naumov, V.B. Physicochemical Parameters and Geochemical Features of Ore-Forming Fluids for Orogenic Gold Deposits Throughout Geological Time. Minerals 2020, 10, 50. [Google Scholar] [CrossRef] [Green Version]
- Ismail, O.S.; Umukoro, G.E. Modelling combustion reactions for gas flaring and its resulting emissions. J. King Saud Univ. Eng. Sci. 2014, 28, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Genna, D.; Gaboury, D.; Azevedo, C.; Jébrak, M. Identifying a magmatic-hydrothermal contribution in Archean auriferous mineralization using pyrite chemistry. In Proceedings of the GSA2020 Connects Online, Boulder, CO, USA, 26–30 October 2020. [Google Scholar]
- Grant, H.L.J.; Hannington, M.D.; Petersen, S.; Frische, M.; Fuchs, S.H. Constraints on the behavior of trace elements in the actively-forming TAG deposit, Mid-Atlantic Ridge, based on LA-ICP-MS analyses of pyrite. Chem. Geol. 2018, 498, 45–71. [Google Scholar] [CrossRef]
- Gerya, T.V.; Perchuk, L.L.; Triboulet, C.; Audren, C.; Sez’ko, A.I. Petrology of the Tumanshet Zonal Metamorphic Complex, Eastern Sayan. Petrology 1997, 5, 503–533. [Google Scholar]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Miner. 2012, 97, 2031–2048. [Google Scholar] [CrossRef]
- Gaboury, D. Parameters for the formation of orogenic gold deposits. Appl. Earth Sci. 2019, 128, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Williams-Jones, A.E.; Bowell, R.J.; Migdisov, A.A. Gold in solution. Elements 2009, 5, 281–287. [Google Scholar] [CrossRef]
- Wagner, T.; Fusswinkel, T.; Wälle, M.; Heinrich, C.A. Microanalysis of fluid inclusions in crustal hydrothermal systems using laser ablation methods. Elements 2016, 12, 323–328. [Google Scholar] [CrossRef]
- Fusswinkel, T.; Wagner, T.; Sakellaris, G. Fluid evolution of the Neoarchean Pampalo orogenic gold deposit (E Finland): Constraints from LA-ICPMS fluid inclusion microanalysis. Chem. Geol. 2017, 450, 96–121. [Google Scholar] [CrossRef]
- Hastie, E.C.G.; Kontak, D.J.; Lafrance, B. Gold remobilization: Insights from gold deposits in the Archean Swayze Greenstone Belt, Abitibi Subprovince, Canada. Econ. Geol. 2020, 115, 241–277. [Google Scholar] [CrossRef]
- Voisey, C.R.; Willis, D.; Tomkins, A.G.; Wilson, C.J.L.; Micklethwaite, S.; Salvemini, F.; Bougoure, J.; Rickard, W.D.A. Aseismic refinement of orogenic gold systems. Econ. Geol. 2020, 115, 33–50. [Google Scholar] [CrossRef]
- Gaboury, D.; Ore Sanchez, C. Electrochemical gold precipitation to explain extensive vertical and lateral mineralization in the world-class Poderosa-Pataz district, Peru. Terra Nova 2020, 32, 97–107. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Akinfiev, N.N.; Borisova, A.Y.; Zotov, A.V.; Kouzmanov, K. Gold speciation and transport in geological fluids: Insights from experiments and physical chemical modeling. Geol. Soc. Spec. Publ. 2014, 402, 9–70. [Google Scholar] [CrossRef]
- Shenberger, S.D.; Barnes, H.L. Solubility of gold in aqueous sulfide solutions from 150–350 °C. Geochim. Cosmochim. Acta 1989, 53, 269–278. [Google Scholar] [CrossRef]
- Stefansson, A.; Seward, T.M. Gold(I) complexing in aqueous sulphide solutions to 500 °C and 500 bar. Geochim. Cosmochim. Acta 2004, 68, 4121–4143. [Google Scholar] [CrossRef]
- Simmons, S.F.; Tutolo, B.M.; Barker, S.L.L.; Goldfarb, R.J.; Robert, F. Hydrothermal Gold Deposition in Epithermal, Carlin, and Orogenic Deposits; Special Publication; Society of Economic Geologists: Littleton, CO, USA, 2020; Volume 23, pp. 823–845. [Google Scholar]
- Pandit, D. Thermodynamic model for hydrothermal sulfide deposition in the paleoproterozoic granite ore system at Malanjkhand, India. Indian J. Geo-Mar. Sci. 2015, 44, 1697–1711. [Google Scholar]
- Gibert, F.; Pascal, M.-L.; Pichavant, M.M. Gold solubility and speciation in hydrothermal solutions: Experimental study of the stability of hydrosulphide complex of gold (AuHS°) at 350 to 450 °C and 500 bars. Geochim. Cosmochim. Acta 1998, 62, 2931–2947. [Google Scholar] [CrossRef] [Green Version]
- Chi, G.X.; Dubé, B.; Williamson, K.; Williams-Jones, A.E. Formation of the Campbell-Red Lake gold deposit by H2O-poor, CO2-dominated fluids. Miner. Depos. 2006, 40, 726–741. [Google Scholar] [CrossRef]
- Mumm, A.S.; Oberthür, T.; Vetter, U.; Blenkinsop, T.G. High CO2 content of fluid inclusions in gold mineralisations in the Ashanti Belt, Ghana: A new category of ore forming fluids? Miner. Depos. 1997, 32, 107–118. [Google Scholar] [CrossRef]
- Klemd, R.; Hirdes, W. Origin of an unusual fluid composition in Early Proterozoic Palaeoplacer and lode-gold deposits in Birimian greenstone terranes of West Africa. S. Afr. J. Geol. 1997, 100, 405–414. [Google Scholar]
- Gaboury, D. Does gold in orogenic deposits come from pyrite in deeply buried carbon-rich sediments? Insight from volatiles in fluid inclusions. Geology 2013, 41, 1207–1210. [Google Scholar] [CrossRef]
- Gaboury, D.; Nabil, H.; Ennaciri, A.; Maacha, L. Structural setting and fluid composition of gold mineralization along the central segment of the Keraf suture, Neoproterozoic Nubian Shield, Sudan: Implications for the source of gold. Int. Geol. Rev. 2020, 1–27. [Google Scholar] [CrossRef]
- Klein, E.L.; Fuzikawa, K. Origin of the CO2-only fluid inclusions in the Palaeoproterozoic Carará vein-quartz gold deposit, Ipitinga Auriferous District, SE-Guiana Shield, Brazil: Implications for orogenic gold mineralisation. Ore Geol. Rev. 2010, 37, 31–40. [Google Scholar] [CrossRef]
- Tarantola, A.; Diamond, L.W.; Stünitz, H. Modification of fluid inclusions in quartz by deviatoric stress I: Experimentally induced changes in inclusion shapes and microstructures. Contrib. Mineral. Petrol. 2010, 160, 825–843. [Google Scholar] [CrossRef]
- Phillips, G.N.; Powell, R. Formation of gold deposits: A metamorphic devolatilization model. J. Metamorph. Geol. 2010, 28, 689–718. [Google Scholar] [CrossRef]
- Crede, L.S.; Evans, K.A.; Rempel, K.U.; Brugger, J.; Etschmann, B.; Bourdet, J.; Reith, F. Revisiting hydrocarbon phase mobilization of Au in the Au–Hg McLaughlin Mine, Geysers/Clear Lake area, California. Ore Geol. Rev. 2020, 117, 103218. [Google Scholar] [CrossRef]
- Emsbo, P.; Koenig, A.E. Transport of Au in petroleum: Evidence from the northern Carlin trend, Nevada. In Mineral Exploration and Research: Digging Deeper, Proceedings of the 9th Biennial SGA Meeting, Dublin, Ireland, 20–23 August 2007; Millpress: Dublin, Ireland, 2007; pp. 695–698. [Google Scholar]
- Fuchs, S.; Schumann, D.; Williams-Jones, A.E.; Vali, H. The growth and concentration of uranium and titanium minerals in hydrocarbons of the Carbon Leader Reef, Witwatersrand Supergroup, South Africa. Chem. Geol. 2015, 393–394, 55–66. [Google Scholar] [CrossRef]
- Fuchs, S.; Williams-Jones, A.E.; Jackson, S.E.; Przybylowicz, W.J. Metal distribution in pyrobitumen of the Carbon Leader Reef, Witwatersrand Supergroup, South Africa: Evidence for liquid hydrocarbon ore fluids. Chem. Geol. 2016, 426, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S.; Schumann, D.; Martin, R.F.; Couillard, M. The extensive hydrocarbon-mediated fixation of hydrothermal gold in the Witwatersrand Basin, South Africa. Ore Geolo. Rev. 2021, 138, 104313. [Google Scholar] [CrossRef]
- Crede, L.S.; Evans, K.A.; Rempel, K.U.; Grice, K.; Sugiyama, I. Gold partitioning between 1-dodecanethiol and brine at elevated temperatures: Implications of Au transport in hydrocarbons for oil-brine ore systems. Chem. Geol. 2019, 504, 28–37. [Google Scholar] [CrossRef]
- Crede, L.S.; Liu, W.; Evans, K.A.; Rempel, K.U.; Testemale, D.; Brugger, J. Crude oils as ore fluids: An experimental in-situ XAS study of gold partitioning between brine and organic fluid from 25 to 250 °C. Geochim. Cosmochim. Acta 2019, 244, 352–365. [Google Scholar] [CrossRef]
- Migdisov, A.; Guo, X.; Williams-Jones, A.; Sun, C.; Vasyukova, O.; Sugiyama, I.; Fuchs, S.; Pearce, K.; Roback, R. Hydrocarbons as ore fluids. Geochem. Perspec. Lett. 2017, 5, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Tagirov, B.; Seward, T.M. Hydrosulfide/sulfide complexes of zinc to 250 °C and the thermodynamic properties of sphalerite. Chem. Geol. 2010, 269, 301–311. [Google Scholar] [CrossRef]
- Sephton, M.A.; Hazen, R.M. On the origins of deep hydrocarbons. Rev. Miner. Geochem. 2013, 75, 449–465. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, S.; Zhang, B.; He, K.; Wang, X. New insight into the kinetics of deep liquid hydrocarbon cracking and its significance. Geofluids 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Tsuzuki, N.; Takeda, M.; Suzuki, M.; Yokoi, K. The kinetic modeling of oil cracking by hydrothermal pyrolysis experiments. Inter. J. Coal Geol. 1999, 39, 227–250. [Google Scholar] [CrossRef]
- Hough, R.M.; Noble, R.R.P.; Reich, M. Natural gold nanoparticles. Ore Geol. Rev. 2011, 42, 55–61. [Google Scholar] [CrossRef]
- Petrella, L.; Thébaud, N.; Fougerouse, D.; Evans, K.; Quadir, Z.; Laflamme, C. Colloidal gold transport: A key to high-grade gold mineralization. Miner. Depos. 2020, 55, 1247–1254. [Google Scholar] [CrossRef]
- McLeish, D.F.; Williams-Jones, A.E.; Vasyukova, O.V.; Clark, J.R.; Board, W.S. Colloidal transport and flocculation are the cause of the hyperenrichment of gold in nature. PNAS 2021, 118, e2100689118. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, M.; Yang, Y.; Mei, Y.; Etschmann, B.; Brugger, J.; Johannessen, B. Colloidal gold in sulphur and citrate-bearing hydrothermal fluids: An experimental study. Ore Geol. Rev. 2019, 114, 103142. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Westgate, T.; Ward, J.; Slater, G.F.; Lacrampe-Couloume, G. Abiogenic formation of alkanes in the Earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 2002, 416, 522–524. [Google Scholar] [CrossRef]
- Etiope, G.; Sherwood Lollar, B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Reeves, E.P.; Fiebig, J. Abiotic Synthesis of Methane and Organic Compounds in Earth’s Lithosphere. Elements 2020, 16, 25–31. [Google Scholar] [CrossRef]
- Doğan, T.; Sumino, H.; Nagao, K.; Notsu, K.; Tuncer, M.K.; Çelik, C. Adjacent releases of mantle helium and soil CO2 from active faults: Observations from the Marmara region of the North Anatolian Fault zone, Turkey. Geochem. Geophys. 2009, 10, 1–11. [Google Scholar]
- Klemperer, S.L.; Kennedy, B.M.; Sastry, S.R.; Makovsky, Y.; Harinarayana, T.; Leech, M.L. Mantle fluids in the Karakoram fault: Helium isotope evidence. Earth Planet. Sci. Lett. 2013, 366, 59–70. [Google Scholar] [CrossRef]
- Buttitta, D.; Caracausi, A.; Chiaraluce, L.; Favara, R.; Morticelli, M.G.; Sulli, A. Continental degassing of helium in an active tectonic setting (northern Italy): The role of seismicity. Sci. Rep. 2020, 10, 162. [Google Scholar] [CrossRef]
- Gaboury, D.; Mackezie, D.; Craw, D. Fluid volatile composition associated with orogenic gold mineralization, Otago Schist, New Zealand: Implications of H2 and C2H6 for fluid evolution and gold source. Ore Geol. Rev. 2021, 133, 104086. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Olivo, G.R.; Teagle, D.A.H.; Craw, D. Sulfide evolution during prograde metamorphism of the Otago and Alpine Schists, New Zealand. Can. Miner. 2010, 48, 1267–1295. [Google Scholar] [CrossRef]
- Finch, E.G.; Tomkins, A.G. Pyrite-pyrrhotite stability in a metamorphic aureole: Implications for orogenic gold genesis. Econ. Geol. 2017, 112, 661–674. [Google Scholar] [CrossRef]
- Thomas, H.V.; Large, R.R.; Bull, S.W.; Maslennikov, V.; Berry, R.F.; Fraser, R.; Froud, S.; Moye, R. Pyrite and pyrrhotite textures and composition in sediments, laminated quartz veins, and reefs at Bendigo gold mine, Australia: Insights for ore genesis. Econ. Geol. 2011, 106, 1–31. [Google Scholar] [CrossRef]
- Large, R.R.; Bull, S.W.; Maslennikov, V.V. A carbonaceous sedimentary source-rock model for Carlin-type and orogenic gold deposits. Econ. Geol. 2011, 106, 331–358. [Google Scholar] [CrossRef]
- Large, R.; Thomas, H.; Craw, D.; Henne, A.; Henderson, S. Diagenetic pyrite as a source for metals in orogenic gold deposits, Otago Schist, New Zealand. New Zealand. J. Geol. Geophys. 2012, 55, 137–149. [Google Scholar] [CrossRef] [Green Version]
- Augustin, J.; Gaboury, D.; Crevier, M. The world-class Wona-Kona gold deposit, Burkina Faso. Ore Geol. Rev. 2016, 78, 667–672. [Google Scholar] [CrossRef]
- Pitcairn, I.K.; Leventis, N.; Beaudoin, G.; Faure, S.; Guilmette, C.; Dubé, B. A metasedimentary source of gold in Archean orogenic gold deposits. Geology 2021, 49, 862–866. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaboury, D.; Genna, D.; Trottier, J.; Bouchard, M.; Augustin, J.; Malcolm, K. The Perron Gold Deposit, Archean Abitibi Belt, Canada: Exceptionally High-Grade Mineralization Related to Higher Gold-Carrying Capacity of Hydrocarbon-Rich Fluids. Minerals 2021, 11, 1066. https://0-doi-org.brum.beds.ac.uk/10.3390/min11101066
Gaboury D, Genna D, Trottier J, Bouchard M, Augustin J, Malcolm K. The Perron Gold Deposit, Archean Abitibi Belt, Canada: Exceptionally High-Grade Mineralization Related to Higher Gold-Carrying Capacity of Hydrocarbon-Rich Fluids. Minerals. 2021; 11(10):1066. https://0-doi-org.brum.beds.ac.uk/10.3390/min11101066
Chicago/Turabian StyleGaboury, Damien, Dominique Genna, Jacques Trottier, Maxime Bouchard, Jérôme Augustin, and Kelly Malcolm. 2021. "The Perron Gold Deposit, Archean Abitibi Belt, Canada: Exceptionally High-Grade Mineralization Related to Higher Gold-Carrying Capacity of Hydrocarbon-Rich Fluids" Minerals 11, no. 10: 1066. https://0-doi-org.brum.beds.ac.uk/10.3390/min11101066