Active Treatment of Contaminants of Emerging Concern in Cold Mine Water Using Advanced Oxidation and Membrane-Related Processes: A Review
Abstract
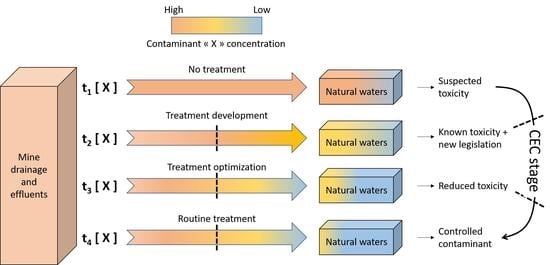
:1. Introduction: Contaminants of Emerging Concern (CECs) in Mine Water
2. Characteristics of CECs
2.1. Background Concentrations of CECs
2.2. Persistent Aquatic Toxicity
3. Treatment of CECs in Mine Water
3.1. Advanced Oxidation Processes (AOPs)
3.2. Ozone Microbubbles
3.3. Membrane Filtration
4. Challenges and Opportunities in Mine Water Treatment in Cold Climates
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laurence, D. Establishing a sustainable mining operation: An overview. J. Clean. Prod. 2011, 19, 278–284. [Google Scholar] [CrossRef]
- Krausmann, F.; Gingrich, S.; Eisenmenger, N.; Erb, K.H.; Haberl, H.; Fischer-Kowalski, M. Growth in global materials use, GDP and population during the 20th century. Ecol. Econ. 2009, 68, 2696–2705. [Google Scholar] [CrossRef]
- Roach, B.; Walker, T.N. Aquatic monitoring programs conducted during environmental impact assessments in Canada: Preliminary assessment before and after weakened environmental regulation. Environ. Monit. Assess. 2017, 189, 109. [Google Scholar] [CrossRef]
- Wolkersdorfer, C.; Nordstrom, D.K.; Beckie, R.D.; Cicerone, D.S.; Elliot, T.; Edraki, M.; Valente, T.; Alves França, S.C.; Kumar, P.; Oyarzún Lucero, R.A.; et al. Guidance for the integrated use of hydrological, geochemical, and isotopic tools in mining operations. Mine Water Environ. 2020, 39, 204–228. [Google Scholar] [CrossRef] [Green Version]
- Sauvé, S.; Desrosiers, M. A review of what is an emerging contaminant. Chem. Cent. J. 2014, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Fairbairn, D.J.; Arnold, W.A.; Barber, B.L.; Kaufenberg, E.F.; Koskinen, W.C.; Novak, P.J.; Rice, P.J.; Swackhamer, D.L. Contaminants of emerging concern: Mass balance and comparison of wastewater effluent and upstream sources in a mixed-use watershed. Environ. Sci. Technol. 2016, 50, 36–45. [Google Scholar] [CrossRef]
- Neculita, C.M.; Rosa, E. A review of the implications and challenges of manganese removal from mine drainage. Chemosphere 2019, 214, 491–510. [Google Scholar] [CrossRef]
- Neculita, C.M.; Coudert, L.; Rosa, E.; Mulligan, C. Future prospects for treating contaminants of emerging concern in water and soils/sediments. In Advanced Nano-Bio Technologies for Water and Soil Treatment; Filip, J., Cajthaml, T., Najmanová, P., Černík, M., Zbořil, R., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 589–605. [Google Scholar]
- Coudert, L.; Bondu, R.; Rakotonimaro, T.V.; Rosa, E.; Guittonny, M.; Neculita, C.M. Treatment of As-rich mine effluents and produced residues stability: Current knowledge and research priorities for gold mining. J. Hazard. Mater. 2020, 386, 121920. [Google Scholar] [CrossRef]
- OECD. New and Emerging Water Pollutants arising from Agriculture; OECD: Paris, France, 2012; p. 49. [Google Scholar]
- Fisheries and Environment Canada. Metal Mining Liquid Effluent Regulations and Guidelines; Report EPS 1-WP-77-1; Fisheries and Environment Canada: Ottawa, ON, Canada, 1977.
- D019. Directive 019 Sur l’Industrie Minière; Government of Quebec: Quebec, QC, Canada, 2005. Available online: https://www.environnement.gouv.qc.ca/milieu_ind/directive019/directive019-2005.pdf (accessed on 18 October 2020).
- CCME Summary Table. Available online: http://st-ts.ccme.ca/en/index.html (accessed on 18 October 2020).
- Minister of Justice. MMER (Metal and Diamond Mining Effluent Regulations); SOR/2002–222; Government of Canada: Ottawa, ON, Canada, 2019.
- Ben Ali, H.E.; Neculita, C.M.; Molson, J.W.; Maqsoud, A.; Zagury, G.J. Performance of passive systems for mine drainage treatment at low temperature and high salinity: A review. Miner. Eng. 2019, 134, 325–344. [Google Scholar] [CrossRef]
- Royer-Lavallée, A.; Neculita, C.M.; Coudert, L. Removal and potential recovery of rare earth elements from mine water. J. Ind. Eng. Chem. 2020, 89, 47–57. [Google Scholar] [CrossRef]
- Costis, S.; Mueller, K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.F. Recovery potential of rare earth elements from mining and industrial residues: A review and case studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- Olías, M.; Cánovas, C.; Basallote, M.D.; Lozano, A. Geochemical behaviour of rare earth elements (REE) along a river reach receiving inputs of acid mine drainage. Chem. Geol. 2018, 493, 468–477. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.K.; Suzuki, T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Plant, J.A.; Kinniburgh, D.G.; Smedley, P.L.; Fordyce, F.M.; Klinck, B.A. Arsenic and selenium. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 17–66. [Google Scholar]
- Gaillardet, J.; Viers, J.; Duprée, B. Trace elements in river waters. In Treatise on Geochemistry; Drever, J.I., Ed.; Pergamon: Oxford, UK, 2003; pp. 225–272. [Google Scholar]
- Shand, P.; Edmunds, W.M. The baseline inorganic chemistry of European groundwaters. In Natural Groundwater Quality; Edmunds, M.W., Shand, P., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2008; pp. 22–58. [Google Scholar]
- Giloteaux, L.; Duran, R.; Casiot, C.; Bruneel, O.; Elbaz-Poulichet, F.; Goni-Urriza, M. Three-year survey of sulfate-reducing bacteria community structure in Carnoulès acid mine drainage (France), highly contaminated by arsenic. FEMS Microbiol. Ecol. 2013, 83, 724–737. [Google Scholar] [CrossRef] [Green Version]
- Majzlan, J.; Plášil, J.; Škoda, R.; Gescher, J.; Kögler, F.; Rusznyak, A.; Küsel, K.; Neu, T.R.; Mangold, S.; Rothe, J. Arsenic-rich acid mine water with extreme arsenic concentration: Mineralogy, geochemistry, microbiology and environmental implications. Environ. Sci. Technol. 2014, 48, 13685–13693. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, F. Selenium geochemistry and health. Ambio 2007, 36, 94–97. [Google Scholar] [CrossRef] [Green Version]
- Flem, B.; Reimann, C.; Fabian, K.; Birke, M.; Filzmoser, P.; Banks, D. Graphical statistics to explore the natural and anthropogenic processes influencing the inorganic quality of drinking water, ground water and surface water. Appl. Geochem. 2018, 88, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Li, L.; Wang, Y. Selenium removal by activated alumina in batch and continuous-flow reactors. Water Environ. Res. 2020, 92, 51–59. [Google Scholar] [CrossRef]
- Sun, Z.; Forsling, W. The degradation kinetics of ethyl-xanthate as a function of pH in aqueous solution. Miner. Eng. 1997, 10, 389–400. [Google Scholar] [CrossRef]
- Xu, Y.; Lay, J.; Korte, F. Fate and effects of xanthates in laboratory freshwater systems. Bull. Environ. Contam. Toxicol. 1988, 41, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Alto, K.; Broderius, S.; Smith, L. Toxicity of Xanthates to Freshwater Fish and Invertebrates; Minnesota Environmental Quality Council: Minneapolis, MN, USA, 1978. [Google Scholar]
- Rostad, C.E.; Schmitt, C.J.; Schumacher, J.G.; Leiker, T.J. An exploratory investigation of polar organic compounds in waters from a lead–zinc mine and mill complex. Water Air Soil Pollut. 2011, 217, 431–443. [Google Scholar] [CrossRef]
- Bach, L.; Nørregaard, R.D.; Hansen, V.; Gustavson, K. Review on Environmental Risk Assessment of Mining Chemicals Used for Mineral; Scientific Report; DCE–Danish Centre for Environment and Energy: Roskilde, Denmark, 2016. [Google Scholar]
- Muzinda, I.; Schreithofer, N. Water quality effects on flotation: Impacts and control of residual xanthates. Miner. Eng. 2018, 125, 34–41. [Google Scholar] [CrossRef]
- Van Dam, R.A.; Harford, A.J.; Lunn, S.A.; Gagnon, M.M. Identifying the cause of toxicity of a saline mine water. PLoS ONE 2014, 9, e106857. [Google Scholar] [CrossRef]
- Pinto, P.X.; Al-Abed, S.R.; Balz, D.A.; Butler, B.A.; Landy, R.B.; Smith, S.J. Bench-scale and pilot-scale treatment technologies for the removal of total dissolved solids from coal mine water: A review. Mine Water Environ. 2016, 35, 94–112. [Google Scholar] [CrossRef]
- Cohen, B.; Lazarovitch, N.; Gilron, J. Upgrading groundwater for irrigation using monovalent selective electrodialysis. Desalination 2018, 431, 126–139. [Google Scholar] [CrossRef]
- Simmons, J.A. Toxicity of major cations and anions (Na+, K+, Ca2+, Cl−, and SO42−) to a macrophyte and an alga. Environ. Toxicol. Chem. 2012, 31, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Bowell, R.J. A Review of Sulfate Removal Options for Mine Waters; SRK Consulting: Cardiff, Wales, UK, 2004; 24p. [Google Scholar]
- Takano, B.; Ohsawa, S.; Glover, R.B. Surveillance of Ruapehu Crater Lake, New Zealand by aqueous polythionates. J. Volcanol. Geoth. Res. 1994, 60, 29–57. [Google Scholar] [CrossRef]
- Johnson, D.B. Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut. 2003, 3, 47–66. [Google Scholar] [CrossRef]
- Range, B.M.K.; Hawboldt, K.A. Adsorption of thiosulphate, trithionate, tetrathionate using biomass ash/char. J. Environ. Chem. Eng. 2018, 6, 5401–5408. [Google Scholar] [CrossRef]
- Miranda-Trevino, J.C.; Pappoe, M.; Hawboldt, K.; Bottaro, C. The importance of thiosalts speciation: Review of analytical methods, kinetics, and treatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2013–2070. [Google Scholar] [CrossRef]
- Range, B.M.K.; Hawboldt, K.A. Removal of thiosalt/sulfate from mining effluents by adsorption and ion exchange. Min. Proc. Ext. Met. Rev. 2019, 40, 79–86. [Google Scholar] [CrossRef]
- Schwartz, M.; Vigneault, B.; McGeer, J. Evaluating the Potential for Thiosalts to Contribute to Toxicity in Mine Effluents; Report presented for Thiosalts Consortium, Project: 602591, Report CANMET-MMSL 06-053 (CR); CANMET Mining and Mineral Sciences Laboratories (CANMET-MMSL): Ottawa, ON, Canada, 2006; p. 101. [Google Scholar]
- Wasserlauf, M.; Dutrizac, J.E. The Chemistry, Generation and Treatment of Thiosalts in Milling Effluents: A Non-Critical Summary of CANMET Investigations 1976–1982; Report: 82-4E; CANMET: Ottawa, ON, Canada, 1982; p. 104. [Google Scholar]
- Schudel, G.; Plante, B.; Bussière, B.; McBeth, J.; Dufour, G. The effect of arctic conditions on the geochemical behaviour of sulphidic tailings. In Proceedings of the Tailings and Mine Waste, Vancouver, BC, Canada,, 17–20 November 2019. [Google Scholar]
- Jouini, M.; Neculita, C.M.; Genty, T.; Benzaazoua, M. Freezing/thawing effects on geochemical behaviour of residues from acid mine drainage passive treatment systems. J. Water Process. Eng. 2020, 33, 101807. [Google Scholar] [CrossRef]
- Chlot, S. Nitrogen and Phosphorus Interactions and Transformations in Cold-Climate Mine Water Recipients. Ph.D. Thesis, Lulea University of Technology, Lulea, Sweden, 2013. [Google Scholar]
- Jermakka, J.; Wendling, L.; Sohlberg, E.; Heinonen, H.; Vikman, M. Potential technologies for the removal and recovery of nitrogen compounds from mine and quarry waters in subarctic conditions. Crit. Rev. Environ. Sci. Technol. 2015, 45, 703–748. [Google Scholar] [CrossRef]
- Mudder, T.I.; Botz, M.M.; Smith, A. Chemistry and Treatment of Cyanidation Wastes, 2nd ed.; Mining Journal Books Limited: London, UK, 2001. [Google Scholar]
- Gould, D.W.; King, M.; Mohapatra, B.R.; Cameron, R.A.; Kapoor, A.; Koren, D.W. A critical review on destruction of thiocyanate in mining effluents. Miner. Eng. 2012, 34, 38–47. [Google Scholar] [CrossRef]
- Johnson, C.A. The fate of cyanide in leach wastes at gold mines: An environmental perspective. Appl. Geochem. 2015, 57, 194–205. [Google Scholar] [CrossRef]
- Marsidi, N.; Hasan, H.A.; Abdullah, S.R.S. A review of biological aerated filters for iron and manganese ions removal in water treatment. J. Water Process. Eng. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Staicu, L.C.; Morin-Crini, N.; Crini, G. Desulfurization: Critical step towards enhanced selenium removal from industrial effluents. Chemosphere 2017, 172, 111–119. [Google Scholar] [CrossRef]
- Golder Associates Inc. Literature Review of Treatment Technologies to Remove Selenium from Mining Influenced Water; Teck Coal Limited: Calgary, AB, Canada, 2009. [Google Scholar]
- Molina, G.C.; Cayo, C.H.; Rodrigues, M.A.S.; Bernardes, A.M. Sodium isopropyl xanthate degradation by advanced oxidation processes. Miner. Eng. 2013, 45, 88–93. [Google Scholar] [CrossRef]
- Novak, L.; Holtze, K.; Wagner, R.; Feasby, G.; Liu, L. Guidance Document for Conducting Toxicity Reduction Evaluation (TRE) Investigations of Canadian Metal Mining Effluents. Prepared ESG International Inc. and SGC Lakefield for TIME Network: Guelph, ON, Canada; Lakefield, ON, Canada, 2002; p. 85. [Google Scholar]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Appl. Geochem. 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Katz, M. The Canadian sulphur problem. Sulphur and Its Inorganic Derivatives in the Canadian Environment; National Research Council of Canada, NRC Associate Committee on Scientific Criteria for Environmental Quality: Ottawa, ON, Canada, 1977; pp. 21–67. [Google Scholar]
- CCME (Canadian Water Quality Guidelines for the Protection of Aquatic Life). Ammonia; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2010; pp. 1–8.
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Removal of ammonia from water by ozone microbubbles. Ind. Eng. Chem. Res. 2013, 52, 318–326. [Google Scholar] [CrossRef]
- Ryskie, S.; Gonzalez-Merchan, C.; Genty, T.; Neculita, C.M. Efficiency of ozone microbubbles for ammonia removal from mine effluents. Miner. Eng. 2019, 145, 106071. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian drinking water quality: Guideline technical document-Nitrate and nitrite. In Water and Air Quality Bureau; Healthy Environments and Consumer Safety: Ottawa, ON, Canada, 2013; p. 128. [Google Scholar]
- Baker, J.A.; Gilron, G.; Chalmers, B.A.; Elphick, J.R. Evaluation of the effect of water type on the toxicity of nitrate to aquatic organisms. Chemosphere 2017, 168, 435–440. [Google Scholar] [CrossRef]
- di Biase, A.; Wei, V.; Kowalski, M.S.; Bratty, M.; Hildebrand, M.; Jabari, P.; Oleszkiewicz, J.A. Ammonia, thiocyanate, and cyanate removal in an aerobic up-flow submerged attached growth reactor treating gold mine wastewater. Chemosphere 2020, 243, 125395. [Google Scholar] [CrossRef] [PubMed]
- D019. Directive 019 Sur l’Industrie Minière; Government of Quebec: Quebec, QC, Canada, 2012. Available online: https://www.environnement.gouv.qc.ca/milieu_ind/directive019/directive019.pdf (accessed on 18 October 2020).
- Shaw, J.R.; Dempsey, T.D.; Chen, C.Y.; Hamilton, J.W.; Folt, C.L. Comparative toxicity of cadmium, zinc, and mixtures of cadmium and zinc to daphnids. Environ. Toxicol. Chem. 2006, 25, 182–189. [Google Scholar] [CrossRef]
- Foudhaili, T.; Jaidi, R.; Neculita, C.M.; Rosa, E.; Triffault-Bouchet, G.; Veilleux, E.; Coudert, L.; Lefebvre, O. Effect of the electrocoagulation process on the toxicity of gold mine effluents: A comparative assessment of Daphnia magna and Daphnia pulex. Sci. Total Environ. 2020, 708, 134739. [Google Scholar] [CrossRef]
- Couture, P.; Pyle, G. Field studies on metal accumulation and effects in fish. In Homeostasis and Toxicology of Essential Metals; Wood, C.M., Farrell, A.P., Brauner, C.J., Eds.; Fish Physiology: Volume 31, Part 1; Academic Press: Cambridge, MA, USA, 2012; pp. 417–473. [Google Scholar]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Okamoto, A.; Yamamuro, M.; Tatarazako, N. Acute toxicity of 50 metals to Daphnia magna. J. Appl. Toxicol. 2015, 35, 824–830. [Google Scholar] [CrossRef]
- Vinot, H.; Larpent, J. Water pollution by uranium ore treatment works. Hydrobiologia 1984, 112, 125–129. [Google Scholar] [CrossRef]
- Yim, J.H.; Kim, K.W.; Kim, S.D. Effect of hardness on acute toxicity of metal mixtures using Daphnia magna: Prediction of acid mine drainage toxicity. J. Hazard. Mater. 2006, 138, 16–21. [Google Scholar] [CrossRef] [PubMed]
- USEPA (US Environmental Protection Agency). Ambient Water Quality Criteria for Zinc; Office of Research and Development: Washington, DC, USA, 1980.
- USEPA. Aquatic Life Ambient Water Quality Criteria for Ammonia–Freshwater; Office of Water: Washington, DC, USA, 2013.
- CCME. Nitrate Ion; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2012; pp. 1–17.
- Eytcheson, S.A.; LeBlanc, G.A. Hemoglobin levels modulate nitrite toxicity to Daphnia magna. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, J.W.; Waller, T.W.; Piwoni, M.D. Acute toxicity of cyanate to Daphnia magna. Bull. Environ. Contam. Toxicol. 1980, 25, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Banks, D.; Younger, P.L.; Arnesen, R.T.; Iversen, E.R.; Banks, S.B. Mine-water chemistry: The good, the bad and the ugly. Environ. Geol. 1997, 32, 157–174. [Google Scholar] [CrossRef]
- Paquin, P.R.; Gorsuch, J.W.; Apte, S.; Batley, G.E.; Bowles, K.C.; Campbell, P.G.; Galvez, F. The biotic ligand model: A historical overview. Comp. Biochem. Phys. C. 2002, 133, 3–35. [Google Scholar] [CrossRef]
- Deleebeeck, N.M.; De Schamphelaere, K.A.; Heijerick, D.G.; Bossuyt, B.T.; Janssen, C.R. The acute toxicity of nickel to Daphnia magna: Predictive capacity of bioavailability models in artificial and natural waters. Ecotoxicol. Environ. Safe. 2008, 70, 67–78. [Google Scholar] [CrossRef]
- Kozlova, T.; Wood, C.M.; McGeer, J.C. The effect of water chemistry on the acute toxicity of nickel to the cladoceran Daphnia pulex and the development of a biotic ligand model. Aquat. Toxicol. 2009, 91, 221–228. [Google Scholar] [CrossRef]
- Persoone, G.; Baudo, R.; Cotman, M.; Blaise, C.; Thompson, K.C.; Moreira-Santos, M.; Han, T. Review on the acute Daphnia magna toxicity test—Evaluation of the sensitivity and the precision of assays performed with organisms from laboratory cultures or hatched from dormant eggs. Knowl. Manag. Aquat. Ec. 2009, 393, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Sivula, L.; Vehniainen, E.R.; Karjalainen, A.K.; Kukkonen, J.V.K. Toxicity of biomining effluents to Daphnia magna: Acute toxicity and transcriptomic biomarkers. Chemosphere 2018, 210, 304–311. [Google Scholar] [CrossRef]
- Bogart, S.J.; Woodman, S.; Steinkey, D.; Meays, C.; Pyle, G.G. Rapid changes in water hardness and alkalinity: Calcite formation is lethal to Daphnia magna. Sci. Total Environ. 2016, 559, 182–191. [Google Scholar] [CrossRef]
- Kang, S.W.; Seo, J.; Han, J.; Lee, J.S.; Jung, J. A comparative study of toxicity identification using Daphnia magna and Tigriopus japonicus: Implications of establishing effluent discharge limits in Korea. Mar. Pollut. Bull. 2011, 63, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Liu, L.; Grondin, L. Toxicity Treatment Evaluation of Mine Final Effluent—Using Chemical and Physical Treatment Methods; Technical Paper; SGS Minerals Services: Lakefield, ON, Canada, 2002. [Google Scholar]
- Lee, S.H.; Kim, I.; Kim, K.W.; Lee, B.T. Ecological assessment of coal mine and metal mine drainage in South Korea using Daphnia magna bioassay. Springer Plus 2015, 4, 518. [Google Scholar] [CrossRef] [Green Version]
- Kratochvil, D. Sustainable water treatment technologies for the treatment of acid mine drainage. In Proceedings of the International Conference on Acid Rock Drainage (ICARD), Ottawa, ON, Canada, 20–26 May 2012. [Google Scholar]
- MEND (Mine Environment Neutral Drainage). Study to Identify BATEA for the Management and Control of Effluent Quality from Mines; MEND Report 3.50.1 prepared by HATCH for MEND Program; MEND: Etobicoke, ON, Canada, 2014; p. 614. [Google Scholar]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hubner, U. Evaluation of advanced oxidation processes for water and wastewater treatment: A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Budaev, S.L.; Batoeva, A.A.; Tsybikova, B.A. Effect of Fenton-like reactions on the degradation of thiocyanate in water treatment. J. Environ. Chem. Eng. 2014, 2, 1907–1911. [Google Scholar] [CrossRef]
- Gonzalez-Merchan, C.; Genty, T.; Bussière, B.; Potvin, R.; Paquin, M.; Benhammadi, M.; Neculita, C.M. Influence of contaminant to hydrogen peroxide to catalyzer molar ratio in the advanced oxidation of thiocyanates and ammonia using Fenton-based processes. J. Environ. Chem. Eng. 2016, 4, 4129–4136. [Google Scholar] [CrossRef]
- Yates, B.J.; Zboril, R.; Sharma, V.K. Engineering aspects of ferrate in water and wastewater treatment: A review. J. Environ. Sci. Heal. 2014, 49, 1603–1614. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.M.; Ghosh, P. Microbubbles-aided water and wastewater purification: A review. Rev. Chem. Eng. 2012, 28, 191–221. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Meshref, M.N.A.; Klamerth, N.; Islam, M.S.; McPhedran, K.N.; Gamal El-Din, M. Understanding the similarities and differences between ozone and peroxone in the degradation of naftenic acids: Comparative performance for potential treatment. Chemosphere 2017, 180, 149–159. [Google Scholar] [CrossRef]
- Gervais, M.; Dubuc, J.; Paquin, M.; Gonzalez-Merchan, C.; Genty, T.; Neculita, C.M. Comparative efficiency of three advanced oxidation processes for thiosalts treatment in mine impacted water. Miner. Eng. 2020, 152, 106349. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Babu, D.S.; Nidheesh, P.V. A review on electrochemical treatment of arsenic from aqueous medium. Chem. Eng. Commun. 2021, 208, 389–410. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, C.P.; Yuan, Y.; Li, F.B. Arsenite oxidation and removal driven by a bio-electro-Fenton process under neutral pH conditions. J. Hazard. Mater. 2014, 275, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Vargas, H.; Dubuc, J.; Wang, Z.; Coudert, L.; Neculita, C.M.; Lefebvre, O. Electro-Fenton beyond the degradation of organics: Treatment of thiosalts in contaminated mine water. Environ. Sci. Technol. 2021, 55, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Merchan, C.; Genty, T.; Bussière, B.; Potvin, R.; Paquin, M.; Benhammadi, M.; Neculita, C.M. Ferrates performance in thiocyanates and ammonia degradation in gold mine effluents. Miner. Eng. 2016, 95, 124–130. [Google Scholar] [CrossRef]
- Gonzalez-Merchan, C.; Genty, T.; Paquin, M.; Gervais, M.; Bussière, B.; Potvin, R.; Neculita, C.M. Influence of ferric iron source on ferrate’s performance and residual contamination during the treatment of gold mine effluents. Miner. Eng. 2018, 127, 61–66. [Google Scholar] [CrossRef]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Oxidation of As(III) to As(V) using ozone microbubbles. Chemosphere 2014, 97, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; He, C.; Yin, R.; Liu, J.; Qiu, T. Ultrasound-enhanced catalytic ozonation oxidation of ammonia in aqueous solution. Int. J. Env. Res. Public Health 2019, 16, 2139. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Wang, B.; Zhu, W.; Tian, K.; Zhang, H. A review on ultrasonic catalytic microbubbles ozonation processes: Properties, hydroxyl radicals generation pathway and potential in application. Catalysts 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Esparza, J.M.; Cueva, N.C.; Pauker, C.S.; Jentzsch, P.V.; Bisesti, F.M. Combined treatment using ozone for cyanide removal from wastewater: A comparison. Revista Internacional de Contaminacion Ambiental 2019, 35, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Vicente, C.; Valverde Flores, J. Removal of lead and zinc from mining effluents by applying air micro-nanobubbles. J. Nanotechnol. 2017, 1, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, H.; Kravchenko, V.; Makedon, V.; Shulha, O.; Oleksandr, S. Preliminary water purification from surfactants and organic compounds through ozone oxidation, intensified by electrical impulses. In Proceedings of the 2019 IEEE 6th International Conference on Energy Smart Systems (ESS), Kyiv, Ukraine, 17–19 April 2019. [Google Scholar]
- Elshorbagy, W.; Chowdhury, R. Water Treatment; InTech: London, UK, 2013; p. 392. [Google Scholar]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Andrés-Mañas, J.A.; Ruiz-Aguirre, A.; Acién, F.G.; Zaragoza, G. Assessment of a pilot system for seawater desalination based on vacuum multi-effect membrane distillation with enhanced heat recovery. Desalination 2018, 443, 110–121. [Google Scholar] [CrossRef]
- Karanasiou, A.; Kostoglou, M.; Karabelas, A. An experimental and theoretical study on separations by vacuum membrane distillation employing hollow-fiber modules. Water 2018, 10, 947. [Google Scholar] [CrossRef] [Green Version]
- Speed, D. Environmental aspects of planarization processes. In Advances in Chemical Mechanical Planarization (CMP); Elsevier: Amsterdam, The Netherlands, 2016; pp. 229–269. [Google Scholar]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. Performance evaluation of reverse osmosis process in the post-treatment of mining wastewaters: Case study of Costerfield mining operations, Victoria, Australia. J. Water Process. Eng. 2020, 34, 101116. [Google Scholar] [CrossRef]
- Watson, I.C.; Morin, O.; Henthorne, L. Desalting Handbook for Planners; Desalination Research and Development Program Report; Bureau of Reclamation: Washington, DC, USA, 2003; p. 72. [Google Scholar]
- Van Geluwe, S.; Braeken, L.; Van der Bruggen, B. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: A review. Water Res. 2011, 45, 3551–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, A.; Andrade, L.; Grossi, L.; Pires, W.; Amaral, M. Acid mine drainage treatment by nanofiltration: A study of membrane fouling, chemical cleaning, and membrane ageing. Sep. Purif. Technol. 2018, 192, 185–195. [Google Scholar] [CrossRef]
- Andrade, L.; Aguiar, A.; Pires, W.; Miranda, G.; Amaral, M. Integrated ultrafiltration-nanofiltration membrane processes applied to the treatment of gold mining effluent: Influence of feed pH and temperature. Sep. Sci. Technol. 2017, 52, 756–766. [Google Scholar] [CrossRef]
- Fujioka, T.; Hoang, A.T.; Okuda, T.; Takeuchi, H.; Tanaka, H.; Nghiem, L.D. Water reclamation using a ceramic nanofiltration membrane and surface flushing with ozonated water. Int. J. Env. Res. Public Health 2018, 15, 799. [Google Scholar] [CrossRef] [Green Version]
- Loganathan, K.; Chelme-Ayala, P.; Gamal El-Din, M. Pilot-scale study on the treatment of basal aquifer water using ultrafiltration, reverse osmosis and evaporation/crystallization to achieve zero-liquid discharge. J. Environ. Manag. 2016, 165, 213–223. [Google Scholar] [CrossRef]
- Sivakumar, M.; Ramezanianpour, M.; O’Halloran, G. Mine water treatment using a vacuum membrane distillation system. APCBEE Proc. 2013, 5, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z. Treatment of heavy-metal wastewater by vacuum membrane distillation: Effect of wastewater properties. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 042019. [Google Scholar] [CrossRef]
- Kolliopoulos, G.; Shum, E.; Papangelakis, V.G. Forward osmosis and freeze crystallization as low energy water recovery processes for a water-sustainable industry. Environ. Process. 2018, 5, 59–75. [Google Scholar] [CrossRef]
- Vital, B.; Bartacek, J.; Ortega-Bravo, J.; Jeison, D. Treatment of acid mine drainage by forward osmosis: Heavy metal rejection and reverse flux of draw solution constituents. Chem. Eng. J. 2018, 332, 85–91. [Google Scholar] [CrossRef]
- Altaee, A.; AlZainati, N. Novel thermal desalination brine reject-sewage effluent salinity gradient for power generation and dilution of brine reject. Energies 2020, 13, 1756. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO)? Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef] [Green Version]
- Ricci, B.C.; Ferreira, C.D.; Aguiar, A.O.; Amaral, M.C. Integration of nanofiltration and reverse osmosis for metal separation and sulfuric acid recovery from gold mining effluent. Sep. Purif. Technol. 2015, 154, 11–21. [Google Scholar] [CrossRef]
- Sierra, C.; Saiz, J.R.Á.; Gallego, J.L.R. Nanofiltration of acid mine drainage in an abandoned mercury mining area. Water Air Soil Pollut. 2013, 224, 1734. [Google Scholar] [CrossRef]
- Meschke, K.; Hofmann, R.; Haseneder, R.; Repke, J.-U. Membrane treatment of leached mining waste–A potential process chain for the separation of the strategic elements germanium and rhenium. Chem. Eng. J. 2020, 380, 122476. [Google Scholar] [CrossRef]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Skilhagen, S.E.; Dugstad, J.E.; Aaberg, R.J. Osmotic power—Power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 2008, 220, 476–482. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]



| Contaminant | Natural Environment Concentration (µg/L) | Mining Environment Concentration (mg/L) | Possible Source of Contamination | Applicable Processing Methods | References |
|---|---|---|---|---|---|
| Mn | 0.004–2 | 0.02–352 | Acid and neutral mine drainage | Co-precipitation Sorption Ion exchange Membrane filtration Oxidation and precipitation Biological | [7,54] |
| Se | <0.01–15 | 1.5–33 | Mine drainage | Membrane filtration Ion exchange Evaporation Co-precipitation Electrocoagulation Photoreduction Adsorption Biological | [27,28,55,56] |
| Xanthates | N/A | 0.2–9 | Flotation process | Advanced oxidation processes Natural degradation | [34,57] |
| Thiosalts | N/A | <700 | Acid mine drainage and mineral processing | Advanced oxidation processes Lime neutralization Biological Natural degradation Electrochemical oxidation Membrane filtration | [43,45,58] |
| Salinity (TDS) | <1000 | <16,000 | Mine drainage | Thermal processes Coagulation-flocculation Membrane filtration Aerobic treatment | [15,59,60] |
| SO42− | 3000–30,000 | 100–5000 | Mine drainage | High density sludge treatment ChemSulphide process Biopaq – Bioteq Sulfatogenic bioreactor Passive treatment Sorption Dispersed alkaline substrates Membrane filtration | [15,61] |
| As | 0.11–2.71 | <130,000 | Mine drainage and hydrometallurgical processes | Co-precipitation Membrane filtration | [9,25] |
| NH3-N | <1000 | 46 | Explosives and cyanide treatment | High pH stripping Membrane filtration Nitrification-denitrification Natural degradation Anammox Electro-oxidation Electrocoagulation Adsorption Advanced oxidation processes | [50,62,63,64] |
| NO2− | <1600 | 4.4 | Explosives Biological treatment | Denitrification Membrane filtration Advanced oxidation processes | [50,64,65] |
| NO3− | <10,000 | 25–100 | Explosives Cyanide treatment Biological treatment | Denitrification Membrane filtration | [50,65,66] |
| CNO− | N/A | 28 | Cyanide treatment | Natural degradation Ozone Biological | [64,67] |
| SCN− | N/A | 168–680 | Cyanide treatment | Ozone Electrochemical oxidation Biological treatment Ferrates Advanced oxidation processes | [52,64] |
| Contaminant | LC50 (mg/L) | EC50 (mg/L) | Test duration (h) | Hardness (mg CaCO3/L) | Temperature (°C) | pH | References |
|---|---|---|---|---|---|---|---|
| Mn | N/A | 9.3 10.27 | 48 | 45 240 | 21 | 6.5–8.5 | [73] |
| Se | N/A | 0.71 | 48 | 72 | 21 | 6.5–8.5 | [73] |
| Na-ethyl xanthate Na-isopropyl xanthate Na-isobutyl xanthate K-amyl xanthate K-pentyl xanthate | N/A | 0.35 3.7 3.6 3.67 3.0 | N/A | N/A | 15 | N/A | [33] |
| S2O32− S4O62− | N/A | 300 750 | N/A | N/A | N/A | N/A | [45] |
| Ca2+ | N/A | 52 560 | 48 | 45 240 | 21 | 6.5–8.5 | [73] |
| Cl− | 2600 | N/A | 24 | N/A | N/A | N/A | [74] |
| Na+ | N/A | 1640 423.13 | 48 | 45 240 | 21 | 6.5–8.5 | [73] |
| K+ | N/A | 93 160.45 | 48 | 45 240 | 21 | 6.5–8.5 | [73] |
| SO42− | 7000 | N/A | 48 | N/A | N/A | N/A | [74] |
| As | N/A | 2.4 7.4 74 | 48 | 72 45 240 | 21 | 6.5–8.5 | [73] |
| Cu | 0.004 0.012 | N/A N/A | 48 48 | 44 ± 4 150 ± 10 | N/A N/A | N/A | [75] |
| Zn | 0.82 0.1 0.655 0.3 1.29 | N/A N/A N/A N/A N/A | 48 24 24 48 48 | N/A 45 196 44 ± 4 150 ± 10 | N/A N/A N/A N/A N/A | N/A | [69] [76] [76] [75] [75] |
| NH3-N | 1.980 | N/A | 24 | N/A | 20 | 7 | [77] |
| NO3− | 2047 | N/A | 48 | 156–172 | N/A | N/A | [78] |
| N-NO2− | N/A | 23 | 48 | N/A | 20 | N/A | [79] |
| CNO− | 18 | N/A | 48 | N/A | N/A | N/A | [80] |
| SCN− | 57.4 0.63–32 | 11.3 N/A | 48 96 | N/A 75 | N/A 8–16 | N/A | [52] |
| Location | Exploitation Type | Parameters | D. magna Toxicity (Toxic Units—TU) | References |
|---|---|---|---|---|
| South Korea | Metal plating plant final effluent | pH = 7.58 DOC = 131.1 mg/L Hardness = 46 mg CaCO3/L Cu dissolved = 0.36 mg/L Cl− = 12,841 mg/L Br− = 2307 mg/L | 6.5 | [88] |
| After treatment by ion exchange | Cl− = 2840 mg/L | <1 | ||
| South Korea | Final effluent of acid rock drainage treatment plant before pH correction | pH = 4.51 DO = 4.11 mg/L DOC = 3.87 mg/L Hardness = 2408 mg CaCO3/L Al dissolved = 31.87 mg/L Cu dissolved ≤ DL Fe dissolved = 13.92 mg/L Zn dissolved = 1.39 mg/L | 5.66 | [88] |
| After pH correction | pH = 7.0 Al dissolved = 0.093 mg/L Cu dissolved = 0.001 Fe dissolved = 0.011 mg/L Zn dissolved = 0.05 mg/L | <1 | ||
| South Korea | Mixed effluents of electronics plant | pH = 8.55 DOC = 139.5 mg/L Hardness = 178.8 mg CaCO3/L Cu total = 1.5624 mg/L Cu dissolved = 0.4793 mg/L | 35.46 | [88] |
| Canada (QC) | Final effluent, LaRonde mine, Agnico Eagle (04-03-1999 to 20-02-2001) | pH = 7.8–9.2 CN total = 0.005–0.36 mg/L CNO− = 3.9–231 mg/L SCN− = 73–293 mg/L NH3-N = 20–88 mg/L SO42− = 1350–2370 mg/L DOC = 35–93.6 mg/L Ca = 470–670 mg/L Cu = 0.02–0.14 mg/L Zn ≤ 0.01–0.26 mg/L | 1–50.5 | [89] |
| Canada (QC) | Final effluent, LaRonde mine, as reported by SGS Lakefield (06-05-2001) | pH = 8.05 TDS = 3320 mg/L CN total = 0.05 mg/L CNO− = 21 mg/L SCN− = 57 mg/L NH3-N = 32.6 mg/L NO2-N = 2.78 mg/L NO3-N = 22 mg/L SO42− = 2200 mg/L DOC = 12.4 mg/L Ca = 713 mg/L Cu = 0.016 mg/L Cu dissolved = 0.011 mg/L Na = 177 mg/L Zn = 0.02 mg/L Zn dissolved ≤ 0.01 mg/L Conductivity = 2710 µmhos/cm Hardness = 1820 mg CaCO3/L DO = 9.4 mg/L | 1.68 | [89] |
| South Korea (Daeduck, Damyang county) | Mine drainage | pH = 6.1 Hardness = 16 mg CaCO3/L Zn = 0.156 mg/L Pb = 0.0402 mg/L Cu ≤ DL Cd = 0.0013 mg/L | 1.6 | [75] |
| South Korea (Myungbong, Boseong county) | Mine drainage | pH = 7.8 Hardness = 50 mg CaCO3/L Zn = 4.797 mg/L Pb = 0.0015 mg/L Cu = 0.0296 mg/L Cd = 0.0176 mg/L | 22.9 | [75] |
| Treatment Type | Industry | Influent | Effluent | Scale | References |
|---|---|---|---|---|---|
| Ozone microbubbles followed by coagulation-flocculation | Gold mine underground water | pH = 6.7 Eh = 445 mV T = 22 °C NH3-N = 22 mg/L NO2− = 5.5 mg/L NO3− = 185 mg/L Cu = 0.154 mg/L Fe = 1.06 mg/L Mn = 3.41 mg/L Zn = 9.6 mg/L | pH = 8.7 Eh = 410 mV T = 22 °C NH3-N = 0.78 mg/L NO2− ≤ DL NO3− = 180 mg/L Cu = 0.01 mg/L Fe = 0.16 mg/L Mn = 0.58 mg/L Zn = 0.2 mg/L | Pilot in continuous flow | [64] |
| Ozone microbubbles | Gold mine process water after cyanide destruction | pH = 9 Eh = 295 mV T = 22 °C NH3-N = 34.6 mg/L NO2− = 6 mg/L NO3− = 48 mg/L CNO− = 15.8 mg/L SCN− = 135 mg/L Total CN = 0.04 mg/L Cu = 1.52 mg/L Fe = 0.5 mg/L Mn = 0.3 mg/L Zn = 0.29 mg/L | pH = 9.3 Eh = 382 mV T = 36 °C NH3-N = 5.4 mg/L NO2− ≤ DL NO3− = 151 mg/L CNO− = 12.5 mg/L SCN− ≤ DL Total CN = 0.05 mg/L Cu ≤ DL Fe ≤ DL Mn = 0.06 mg/L Zn ≤ DL | Pilot in batch mode | [64] |
| O3 + H2O2 | Gold mine process water | CN = 172.5 mg/L pH = 11 Turbidity = 56 NTU TOC = 137.8 mg/L DOC = 496 mg/L | CN = 0.08 mg/L pH = 7 Turbidity = 68 NTU TOC = 24.5 mg/L DOC = 118.5 mg/L | Laboratory | [110] |
| Air micro-nanobubbles | Mine effluent | T = 21 °C pH = 11.99 Turbidity = 170 NTU Conductivity = 4.06 mS/cm DO ≤ 0.1 mg/L TS = 4740 mg/L TSS = 251 mg/L Pb = 51.3 mg/L Zn = 17.601 mg/L | T = 22 °C pH = 11.72 Turbidity = 15.93 NTU Conductivity = 2.39 mS/cm DO = 8.53 mg/L TS = 4120 mg/L TSS = 31 mg/L Pb = 0.98 mg/L Zn = 0.128 mg/L | Laboratory | [111] |
| O3 + ultrasound | Synthetic water with surfactant | pH = 8.3 Sodium lauryl sulfate = 100 mg/L | pH = 8.3 Sodium lauryl sulfate = 16.7 mg/L | Laboratory | [112] |
| Ozone microbubbles | Synthetic water with As(III) | pH = 7 As(III) = 200 µg/L As(V) ≤ DL | pH = 7 As(III) ≤ DL As(V) = 200 µg/L | Laboratory | [107] |
| Process | Separation Mechanism | Material/Type | Typical Transmembrane Pressure | Process | Separation Mechanism | Material/Type |
|---|---|---|---|---|---|---|
| Microfiltration (MF) | Separation by sieving through macropores (>50 nm) | Polymer and inorganic/Porous | 10–100 | 90–99+ | Removal of suspended matter and coarse colloidal particles including microorganisms | Pore size Exclusion at the membrane interface |
| Ultrafiltration (UF) | Separation by sieving through mesopores (2–50 nm) | Polymer and inorganic/Porous | 50–300 | 85–95+ | Removal of coarse molecules in solution and colloidal particles in suspension including bacteria and macromolecules such as proteins | |
| Nanofiltration (NF) | Separation by a combination of charge rejection, diffusion of solubility, and sieving through micropores (<2 nm) | Polymer and inorganic/Dense | 200–1500 | 75–90+ | Removal of multivalent ions and specific charged or polar molecules | Solution Diffusion through the membrane |
| Reverse osmosis (RO) | Separation based on the difference in solubility and diffusion rate of water and solutes | Polymer/Dense | 500–8000 | 60–90 | Removal of low molecular weight compounds such as inorganic ions |
| Treatment | Industry | Influent | Effluent (Permeate) | Scale | References |
|---|---|---|---|---|---|
| MF + NF + RO | Gold mine pressure oxidation process water effluent | pH = 1.46 EC = 28.07 mS/cm TSS = 571 mg/L TDS = 23 973 mg/L Cu = 156.8 mg/L Co = 40.01 mg/L Ni = 256.8 mg/L Ca = 487.6 mg/L Mg = 2561 mg/L Fe = 436.5 mg/L Mn = 105.5 mg/L Al = 348 mg/L As = 34.6 mg/L Total Acidity = 10.28 g CaCO3/L Free Acidity = 6.89 g CaCO3/L SO42− = 21 480 mg/L | pH = 2.56 EC = 0.79 mS/cm TSS = 0 mg/L TDS = 192 mg/L Cu = 0.22 mg/L Co = 0.09 mg/L Ni = 1.24 mg/L Ca = 1.61 mg/L Mg = 11.96 mg/L Fe = 0.56 mg/L Mn = 0.13 mg/L Al = 0.63 mg/L As = 2 mg/L - - SO42− = 270 mg/L | Pilot | [132] |
| NF | Acid mine drainage from abandoned mercury mine | Fe = 515 mg/L Al = 23 mg/L As = 6 mg/L Hg = 2.3 µg/L SO42− = 2300 mg/L pH = 2.47 ORP = 592 mV DO = 3.3 mg/L | Fe = 7.5 mg/L Al = 1.7 mg/L As = 0.08 mg/L - SO42− = 245 mg/L - - - | Pilot | [133] |
| MF + NF | Acidic bioleaching mining waste process | Co = 4.04 mg/L Ge = 1.33 mg/L Mo = 14.30 mg/L Re = 3.26 mg/L Cu = 54 mg/L Fe = 1980 mg/L Zn = 720 mg/L | Co = 0.04 mg/L Ge = 1.18 mg/L Mo = 0.39 mg/L Re = 2.96 mg/L Cu = 0.74 mg/L Fe = 15 mg/L Zn = 7.8 mg/L | Laboratory | [134] |
| RO | Final effluent of antimony mine in operation | Turbidity = 39.4 NTU TDS = 7910 mg/L Sb = 49.8 mg/L As = 0.038 mg/L Ni = 0.052 mg/L Zn = 0.052 mg/L Fe = 0.05 mg/L Cd = 0.0001 mg/L Cr = 0.001 mg/L Cu = 0.002 mg/L Pb = 0.001 mg/L | Turbidity = 1 NTU TDS = 218 mg/L Sb = 0.1 mg/L As = 0.001 mg/L Ni = 0.001 mg/L Zn = 0.001 mg/L Fe = 0.05 mg/L Cd = 0.0001 mg/L Cr = 0.001 mg/L Cu = 0.001 mg/L Pb = 0.001 mg/L | Industrial | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryskie, S.; Neculita, C.M.; Rosa, E.; Coudert, L.; Couture, P. Active Treatment of Contaminants of Emerging Concern in Cold Mine Water Using Advanced Oxidation and Membrane-Related Processes: A Review. Minerals 2021, 11, 259. https://0-doi-org.brum.beds.ac.uk/10.3390/min11030259
Ryskie S, Neculita CM, Rosa E, Coudert L, Couture P. Active Treatment of Contaminants of Emerging Concern in Cold Mine Water Using Advanced Oxidation and Membrane-Related Processes: A Review. Minerals. 2021; 11(3):259. https://0-doi-org.brum.beds.ac.uk/10.3390/min11030259
Chicago/Turabian StyleRyskie, Sébastien, Carmen M. Neculita, Eric Rosa, Lucie Coudert, and Patrice Couture. 2021. "Active Treatment of Contaminants of Emerging Concern in Cold Mine Water Using Advanced Oxidation and Membrane-Related Processes: A Review" Minerals 11, no. 3: 259. https://0-doi-org.brum.beds.ac.uk/10.3390/min11030259