Chemical Composition and Genesis Implication of Garnet from the Laoshankou Fe-Cu-Au Deposit, the Northern Margin of East Junggar, NW China
Abstract
:1. Introduction
2. Regional and Deposit Geology
2.1. Regional Geology
2.2. Deposit Geology
3. Samples and Analytical Methods
3.1. Electron Microprobe Analysis
3.2. LA-ICP-MS Analysis
4. Results
4.1. Garnet Petrography
4.2. Major Elements of Garnet by EMPA
4.3. Trace and Rare Earth Elements of Garnet by LA-ICP-MS
4.3.1. Trace Elements
4.3.2. Rare Earth Elements
5. Discussion
5.1. Garnet Substitution Mechanisms
5.2. Physicochemical Conditions of Fluids
5.2.1. Oxygen Fugacity (ƒO2)
5.2.2. pH, Temperature and Salinity
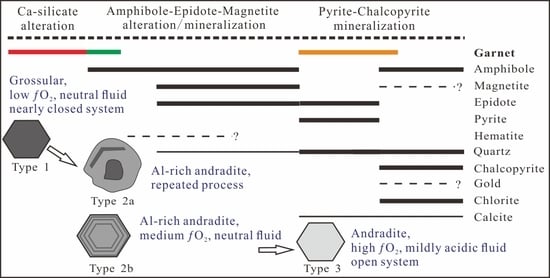
5.3. Constraints on Garnet Formation and Fluid Evolution
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spengler, D.; Obata, M.; Hirajima, T.; Ottolini, L.; Ohfuji, H.; Tamura, A.; Arai, S. Exsolution of garnet and clinopyroxene from high-Al pyroxenes in Xugou peridotite, Eastern China. J. Petrol. 2012, 53, 1477–1504. [Google Scholar] [CrossRef]
- Tian, Z.D.; Leng, C.B.; Zhang, X.C.; Zafar, T.; Zhang, L.J.; Hong, W.; Lai, C.K. Chemical composition, genesis and exploration implication of garnet from the Hongshan Cu-Mo skarn deposit, SW China. Ore Geol. Rev. 2019, 112, 103016. [Google Scholar] [CrossRef]
- Meinert, L.D.; Dipple, G.M.; Nicolescu, S. World skarn deposits. Econ. Geol. 2005, 100th Anniversary Volume, 299–336. [Google Scholar]
- Bea, F.; Montero, P.; Garuti, G.; Zacharini, F. Pressure-dependence of rare earth element distribution in amphibolite-and granulite-grade garnets: A LA-ICP-MS Study. Geostand. Newslett 1997, 21, 253–270. [Google Scholar] [CrossRef]
- Beyer, C.; Frost, D.J.; Miyajima, N. Experimental calibration of a garnet–clinopyroxene geobarometer for mantle eclogites. Contrib. Mineral. Petrol. 2015, 169, 18. [Google Scholar] [CrossRef]
- Caddick, M.J.; Kohn, M.J. Garnet: Witness to the evolution of destructive plate boundaries. Elements 2013, 9, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Zhou, T.F.; White, N.C.; Zhang, L.J.; Fan, Y.; Wang, F.Y.; Chen, X.F. The formation and trace elements of garnet in the skarn zone from the Xinqiao Cu-S-Fe-Au deposit, Tongling ore district, Anhui Province, Eastern China. Lithos 2018, 302, 467–479. [Google Scholar] [CrossRef]
- Blichert-Toft, J.; Albarede, F.; Kornprobst, J. Lu-Hf isotope systematics of garnet pyroxenites from Beni Bousera, Morocco: Implications for basalt origin. Science 1999, 283, 1303–1306. [Google Scholar] [CrossRef]
- Scherer, E.E.; Cameron, K.L.; Blichert-Toft, J. Lu–Hf garnet geochronology: Closure temperature relative to the Sm–Nd system and the effects of trace mineral inclusions. Geochim. Cosmochim. Acta 2000, 64, 3413–3432. [Google Scholar] [CrossRef]
- Grew, E.S.; Marsh, J.H.; Yates, M.G.; Lazic, B.; Armbruster, T.; Locock, A.; Bell, S.W.; Dyar, M.D.; Bernhardt, H., Jr.; Medenbach, O. Menzerite-(Y), a new species,{(Y, REE)(Ca, Fe2+)2}[(Mg, Fe2+)(Fe3+, Al)](Si3)O12, from a felsic granulite, Parry Sound, Ontario, and a new garnet end-member,{Y2Ca}[Mg2](Si3)O12. Can. Mineral. 2010, 48, 1171–1193. [Google Scholar] [CrossRef]
- Fei, X.H.; Zhang, Z.C.; Cheng, Z.G.; Santosh, M. Factors controlling the crystal morphology and chemistry of garnet in skarn deposits: A case study from the Cuihongshan polymetallic deposit, Lesser Xing’an Range, NE China. Am. Mineral. 2019, 104, 1455–1468. [Google Scholar] [CrossRef]
- Gaspar, M.; Knaack, C.; Meinert, L.D.; Moretti, R. REE in skarn systems: A LA-ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmochim. Acta 2008, 72, 185–205. [Google Scholar] [CrossRef]
- Einaudi, M.T.; Burt, D.M. Introduction, terminology, classification, and composition of skarn deposits. Econ. Geol. 1982, 77, 745–754. [Google Scholar] [CrossRef]
- Jamtveit, B.; Wogelius, R.A.; Fraser, D.G. Zonation patterns of skarn garnets: Records of hydrothermal system evolution. Geology 1993, 21, 113–116. [Google Scholar] [CrossRef]
- Park, C.; Choi, W.; Kim, H.; Park, M.H.; Kang, I.M.; Lee, H.S.; Song, Y. Oscillatory zoning in skarn garnet: Implications for tungsten ore exploration. Ore Geol. Rev. 2017, 89, 1006–1018. [Google Scholar] [CrossRef]
- Park, C.; Song, Y.; Kang, I.M.; Shim, J.; Chung, D.; Park, C.S. Metasomatic changes during periodic fluid flux recorded in grandite garnet from the Weondong W-skarn deposit, South Korea. Chem. Geol. 2017, 451, 135–153. [Google Scholar] [CrossRef]
- Dziggel, A.; Wulff, K.; Kolb, J.; Meyer, F.M.; Lahaye, Y. Significance of oscillatory and bell-shaped growth zoning in hydrothermal garnet: Evidence from the Navachab gold deposit, Namibia. Chem. Geol. 2009, 262, 262–276. [Google Scholar] [CrossRef]
- Zhai, D.G.; Liu, J.J.; Zhang, H.Y.; Wang, J.P.; Su, L.; Yang, X.A.; Wu, S.H. Origin of oscillatory zoned garnets from the Xieertala Fe–Zn skarn deposit, northern China: In situ LA–ICP-MS evidence. Lithos 2014, 190, 279–291. [Google Scholar] [CrossRef]
- Kim, H.S. Deformation-induced garnet zoning. Gondwana Res. 2006, 10, 379–388. [Google Scholar] [CrossRef]
- Konrad-Schmolke, M.; O’Brien, P.J.; de Capitani, C.; Carswell, D.A. Garnet growth at high-and ultra-high pressure conditions and the effect of element fractionation on mineral modes and composition. Lithos 2008, 103, 309–332. [Google Scholar] [CrossRef]
- Smit, M.A.; Scherer, E.E.; Mezger, K. Lu–Hf and Sm–Nd garnet geochronology: Chronometric closure and implications for dating petrological processes. Earth Planet. Sci. Lett. 2013, 381, 222–233. [Google Scholar] [CrossRef]
- Fan, X.J.; Wang, X.D.; Lü, X.B.; Wei, W.; Chen, W. Garnet composition as an indicator of skarn formation: LA-ICP-MS and EPMA studies on oscillatory zoned garnets from the Haobugao skarn deposit, Inner Mongolia, China. Geol. J. 2019, 54, 1976–1992. [Google Scholar] [CrossRef]
- Jahn, B.M.; Wu, F.Y.; Chen, B. Massive granitoid generation in Central Asia: Nd isotope evidence and implication for continental growth in the Phanerozoic. Episodes 2000, 23, 82–92. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.J.; Pirajno, F.; Wu, G.; Qi, J.P.; Xiong, X.L. Epithermal deposits in North Xinjiang, NW China. Int. J. Earth Sci. 2011, 101, 889–917. [Google Scholar] [CrossRef]
- Sengor, A.M.C.; Natalin, B.A.; Burtman, V.S. Evolution of the Altaid tectonic collage and Paleozoic crustal growth in Eurasia. Nature 1993, 364, 299–307. [Google Scholar] [CrossRef]
- Liang, P.; Chen, H.Y.; Han, J.S.; Wu, C.; Zhang, W.F.; Xu, D.R.; Lai, C.K.; Kyser, K. Iron oxide-copper-gold mineralization of the Devonian Laoshankou deposit (Xinjiang, NW China) in the Central Asian Orogenic Belt. Ore Geol. Rev. 2019, 104, 628–655. [Google Scholar] [CrossRef]
- Pirajno, F.; Mao, J.; Zhang, Z.; Zhang, Z.; Chai, F. The association of mafic–ultramafic intrusions and A-type magmatism in the Tian Shan and Altay orogens, NW China: Implications for geodynamic evolution and potential for the discovery of new ore deposits. J. Asian Earth Sci. 2008, 32, 165–183. [Google Scholar] [CrossRef]
- Wei, Q.F. Feature of the explosive breccia rocks of the Laoshankou Au-Cu deposit and its geological signifcance, Qinghe, Xinjiang. Geol. Explor. Non-Free. Met. 1997, 6, 331–334, (In Chinese with English Abstract). [Google Scholar]
- Cheng, J. The geological characteristics and genesis of IV ore block in Laoshankou gold-copper-iron diggings, Qinghe County, Xinjiang. Xinjiang Nonferrous Met. 2004, S1, 22–25. (In Chinese) [Google Scholar]
- Li, T.D.; Wang, Z.J. Mineralization characteristics of the IV part of Laoshankou Fe–Cu–Au deposit in Qinghe County, Xinjiang. Xinjiang Nonferrous Met. 2009, 2, 19–25. (In Chinese) [Google Scholar]
- Lü, S.J. Metallogenic Mechanism of Laoshankou Fe-Cu-Au Deposit in Qinghe Country, Xinjiang. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2012. (In Chinese with English Abstract). [Google Scholar]
- Li, Q.; Lü, S.J.; Yang, F.Q.; Geng, X.X.; Chai, F.M. Geological characteristics and genesis of the Laoshankou Fe-Cu-Au deposit in Junggar, Xinjiang, Central Asian Orogenic Belt. Ore Geol. Rev. 2015, 68, 59–78. [Google Scholar] [CrossRef]
- Liang, P.; Chen, H.; Wu, C.; Xie, Y. Pyrite and magnetite Re–Os isotope systematics at the Laoshankou Fe–Cu–Au deposit in the northern margin of the East Junggar terrane, NW Xinjiang, China: Constraints on the multistage mineralization and metal sources. Geol. J. 2020, 55, 4265–4278. [Google Scholar] [CrossRef]
- Zonenshaĭn, L.P.; Kuzʹmin, M.I.; Natapov, L.M. Geology of the USSR: A Plate-Tectonic Synthesis; American Geophysical Union: Washington, DC, USA, 1990; p. 227. [Google Scholar]
- Bai, M. Ertix active fault zone. Xinjiang Geol. 1996, 14, 127–134, (In Chinese with English Abstract). [Google Scholar]
- Liu, F.; Wang, Z.; Lin, W.; Chen, K.; Jiang, L.; Wang, Q. Structure deformation and tectonic significance of Erqis fault zone in the southern margin of Chinese Altay. Acta Petrol. Sin. 2013, 29, 1811–1824, (In Chinese with English Abstract). [Google Scholar]
- Laurent-Charvet, S.; Charvet, J.; Shu, L.; Ma, R.; Lu, H. Palaeozoic late collisional strike-slip deformations in Tianshan and Altay, Eastern Xinjiang, NW China. Terra Nova 2002, 14, 249–256. [Google Scholar] [CrossRef]
- Laurent-Charvet, S.; Charvet, J.; Monie, P.; Shu, L. Late Paleozoic strike-slip shear zones in eastern Central Asia (NW China): New structural and geochronological data. Tectonics 2003, 22, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Buslov, M.; Fujiwara, Y.; Iwata, K.; Semakov, N. Late paleozoic-early Mesozoic geodynamics of Central Asia. Gondwana Res. 2004, 7, 791–808. [Google Scholar] [CrossRef]
- Shi, L.B.; Lin, C.Y.; Chen, X.D.; Zhang, X.O.; Bai, M. The Feature of Fault Rocks and Ancient Seismic Source for the Ertai Fault, Xinjaing. Acta Seismol. Sin. 1997, 19, 291–298. (In Chinese) [Google Scholar]
- Bai, M. Structural Features in the Mid-south Segment of Fuyun, Xinjiang, Earthquake Fault Zone. Inland Earthq. 2002, 16, 126–135, (In Chinese with English Abstract). [Google Scholar]
- Compiling Group of Stratigraphic Chart, X. Stratigraphic Chart in the Northwest (Xinjiang Fascicule); Geological Publishing House: Beijing, China, 1983; p. 496. [Google Scholar]
- Xue, C.J.; Zhao, Z.F.; Wu, G.G.; Dong, L.H.; Feng, J.; Zhang, Z.C.; Zhou, G.; Chi, G.X.; Gao, J.G. The multiperiodic superimposed porphyry copper mineralization in Central Asian Tectonic Region: A case study of geology, geochemistry and chronology of Halasu copper deposit, Southeastern Altai, China. Earth Sci. Front. 2010, 17, 53–82, (In Chinese with English Abstract). [Google Scholar]
- Tong, Y. Geochronology, Origin of the Late Paleozoic Granitoids from the Altai Orogen in China and Their Geological Significance. Ph.D. Thesis, Chinese Academy of Geological Sciences, Beijing, China, 2006. (In Chinese with English Abstract). [Google Scholar]
- Lu, S.J.; Yang, F.Q.; Chai, F.M.; Zhang, X.B.; Jiang, L.P.; Liu, F.; Zhang, Z.X.; Geng, X.X.; Ouyang, L.J. Zircon U-Pb dating for intrusions in Laoshankou ore district in northern margin of East Junggar and their significances. Geol. Rev. 2012, 58, 149–164, (In Chinese with English Abstract). [Google Scholar]
- Li, H.Q.; Chen, F.W. Isotopic Geochronology of Regional Mineralization in Xinjiang, China; Geological Publishing House: Beijing, China, 2004; p. 361. [Google Scholar]
- Xu, J.F.; Mei, H.J.; Yu, X.Y.; Bai, Z.H.; Niu, H.C.; Chen, F.R.; Zhen, Z.P.; Wang, Q. Adakites related to subduction in the northern margin of Junggar arc for the Late Paleozoic: Products of slab melting. Chin. Sci. Bull. 2001, 46, 1312–1316. [Google Scholar] [CrossRef]
- Long, X.P.; Yuan, C.; Sun, M.; Safonova, I.; Xiao, W.J.; Wang, Y.J. Geochemistry and U–Pb detrital zircon dating of Paleozoic graywackes in East Junggar, NW China: Insights into subduction–accretion processes in the southern Central Asian Orogenic Belt. Gondwana Res. 2012, 21, 637–653. [Google Scholar] [CrossRef]
- Xu, X.W.; Jiang, N.; Li, X.H.; Qu, X.; Yang, Y.H.; Mao, Q.; Wu, Q.; Zhang, Y.; Dong, L.H. Tectonic evolution of the East Junggar terrane: Evidence from the Taheir tectonic window, Xinjiang, China. Gondwana Res. 2013, 24, 578–600. [Google Scholar] [CrossRef]
- Liang, P.; Chen, H.; Hollings, P.; Wu, C.; Xiao, B.; Bao, Z.; Xu, D. Geochronology and geochemistry of igneous rocks from the Laoshankou district, North Xinjiang: Implications for the Late Paleozoic tectonic evolution and metallogenesis of East Junggar. Lithos 2016, 266, 115–132. [Google Scholar] [CrossRef]
- Liang, P.; Chen, H.; Han, J.; Wu, C.; Zhang, W.; Zhao, L.; Wang, Y. The Early Carboniferous tectonic transition in the northern margin of East Junggar: Constrains from geochronology and geochemistry of alkali granites. Geotecton. Metallog. 2017, 41, 202–221, (In Chinese with English Abstract). [Google Scholar]
- Han, B.F.; Ji, J.Q.; Song, B.; Chen, L.H.; Zhang, L. Late Paleozoic vertical growth of continental crust around the Junggar Basin, Xinjiang, China (Part I): Timing of post-collisional plutonism. Acta Petrol. Sin. 2006, 22, 1077–1086, (In Chinese with English Abstract). [Google Scholar]
- Lu, S.J.; Zhang, Z.X.; Yang, F.Q.; Chai, F.M.; Zhang, X.B.; Liu, F.; Jiang, L.P.; Geng, X.X. Ore-forming fluids and mineralization mechanism of Laoshankou Fe-Cu-Au deposit in northern margin of Junggar. Miner. Depos. 2012, 31, 517–534, (In Chinese with English Abstract). [Google Scholar]
- Chai, F.M.; Yang, F.Q.; Liu, F.; Geng, X.X.; Lu, S.J.; Jiang, L.P.; Zang, M.; Chen, B. Geochronology and genesis of volcanic rocks in Beitashan Formation at the northern margin of the Junggar, Xinjiang. Acta Petrol. Sin. 2012, 28, 2183–2198, (In Chinese with English Abstract). [Google Scholar]
- Liang, P.; Chen, H.; Wu, C.; Liu, Z. Geochemistry, geochronology and oxygen fugacity of volcanic and intrusive rocks from the Laoshankou Fe-Cu-Au deposit in the northern margin of East Junggar, NW China. Earth Sci. Front. 2018, 25, 96–118, (In Chinese with English Abstract). [Google Scholar]
- Droop, G. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.S.; Hu, Z.C.; Gao, S.; Günther, D.; Xu, J.; Gao, C.G.; Chen, H.H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Whitney, D.L.; Evans, B.W. Abbreviations for names of rock-forming minerals. Am. Mineral. 2010, 95, 185–187. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Carlson, W.D. Rates and mechanism of Y, REE, and Cr diffusion in garnet. Am. Mineral. 2012, 97, 1598–1618. [Google Scholar] [CrossRef]
- Jaffe, H.W. The role of yttrium and other minor elements in the garnet group. Am. Mineral. 1951, 36, 133–155. [Google Scholar]
- Enami, M.; Bolin, C.; Yoshida, T.; Kawabe, I. A mechanism for Na incorporation in garnet: An example from garnet in orthogneiss from the Su-Lu terrane, eastern China. Am. Mineral. 1995, 80, 475–482. [Google Scholar] [CrossRef]
- Ismail, R.; Ciobanu, C.L.; Cook, N.J.; Teale, G.S.; Giles, D.; Mumm, A.S.; Wade, B. Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide–copper–gold deposit, Yorke Peninsula, South Australia. Lithos 2014, 184, 456–477. [Google Scholar] [CrossRef]
- Quartieri, S.; Chaboy, J.; Antonioli, G.; Geiger, C. XAFS characterization of the structural site of Yb in synthetic pyrope and grossular garnets. II: XANES full multiple scattering calculations at the Yb LI-and LIII-edges. Phys. Chem. Miner. 1999, 27, 88–94. [Google Scholar] [CrossRef]
- Quartieri, S.; Antonioli, G.; Geiger, C.; Artioli, G.; Lottici, P. XAFS characterization of the structural site of Yb in synthetic pyrope and grossular garnets. Phys. Chem. Miner. 1999, 26, 251–256. [Google Scholar] [CrossRef]
- Sepidbar, F.; Mirnejad, H.; Li, J.W.; Wei, C.J.; George, L.L.; Burlinson, K. Mineral geochemistry of the Sangan skarn deposit, NE Iran: Implication for the evolution of hydrothermal fluid. Geochemistry 2017, 77, 399–419. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Li, D.; Lin, H. U-Pb geochronology and geochemistry of U-rich garnet from the giant Beiya gold-polymetallic deposit in SW China: Constraints on skarn mineralization process. Minerals 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.P.; Henderson, P.; Jeffries, T.E.R.; Long, J.; Williams, C.T. The rare earth elements and uranium in garnets from the Beinn an Dubhaich Aureole, Skye, Scotland, UK: Constraints on processes in a dynamic hydrothermal system. J. Petrol. 2004, 45, 457–484. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shao, Y.J.; Wu, C.D.; Chen, H.Y. LA-ICP-MS trace element geochemistry of garnets: Constraints on hydrothermal fluid evolution and genesis of the Xinqiao Cu–S–Fe–Au deposit, eastern China. Ore Geol. Rev. 2017, 86, 426–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Shao, Y.; Li, H. Fingerprinting the hydrothermal fluid characteristics from LA-ICP-MS trace element geochemistry of garnet in the Yongping Cu deposit, SE China. Minerals 2017, 7, 199. [Google Scholar] [CrossRef]
- Jamtveit, B.; Ragnarsdottir, K.V.; Wood, B.J. On the origin of zoned grossular-andradite garnets in hydrothermal systems. Eur. J. Mineral. 1995, 7, 1399–1410. [Google Scholar] [CrossRef]
- Ren, T.; Zhong, H.; Zhang, X.; Zhu, W. REE geochemistry of garnets from the Langdu skarn copper deposit. Earth Sci. Front. 2010, 17, 348–358. [Google Scholar]
- Pei, L.; Chao, W.; Xia, H.; Yuling, X. Textures and geochemistry of magnetite: Indications for genesis of the Late Paleozoic Laoshankou Fe-Cu-Au deposit, NW China. Ore Geol. Rev. 2020, 124, 103632. [Google Scholar]
- Huang, X.W.; Boutroy, É.; Makvandi, S.; Beaudoin, G.; Corriveau, L.; De Toni, A.F. Trace element composition of iron oxides from IOCG and IOA deposits: Relationship to hydrothermal alteration and deposit subtypes. Miner. Depos. 2019, 54, 525–552. [Google Scholar] [CrossRef] [Green Version]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid-rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Allen, D.E.; Seyfried, W., Jr. REE controls in ultramafic hosted MOR hydrothermal systems: An experimental study at elevated temperature and pressure. Geochim. Cosmochim. Acta 2005, 69, 675–683. [Google Scholar] [CrossRef]
- Mayanovic, R.A.; Anderson, A.J.; Bassett, W.A.; Chou, I.M. On the formation and structure of rare-earth element complexes in aqueous solutions under hydrothermal conditions with new data on gadolinium aqua and chloro complexes. Chem. Geol. 2007, 239, 266–283. [Google Scholar] [CrossRef]
- Giuliani, G.; Cheilletz, A.; Mechiche, M. Behaviour of REE during thermal metamorphism and hydrothermal infiltration associated with skarn and vein-type tungsten ore bodies in central Morocco. Chem. Geol. 1987, 64, 279–294. [Google Scholar] [CrossRef]
- Vander Auwera, J.; André, L. Trace elements (REE) and isotopes (O, C, Sr) to characterize the metasomatic fluid sources: Evidence from the skarn deposit (Fe, W, Cu) of Traversella (Ivrea, Italy). Contrib. Mineral. Petrol. 1991, 106, 325–339. [Google Scholar] [CrossRef]
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Zhang, Y. Genesis of Xinqiao Cu-S-Fe Deposit, Tongling, Anhui Province, China. Ph.D. Thesis, Central South University, Changsha, China, 2015. (In Chinese with English Abstract). [Google Scholar]
- Fei, X.H.; Zhang, Z.C.; Cheng, Z.G.; Santosh, M.; Jin, Z.L.; Wen, B.B.; Li, Z.X.; Xu, L.J. Highly differentiated magmas linked with polymetallic mineralization: A case study from the Cuihongshan granitic intrusions, Lesser Xing’an Range, NE China. Lithos 2018, 302, 158–177. [Google Scholar] [CrossRef]
- Harlov, D.; Austrheim, H. Metasomatism and the Chemical Transformation of Rock: The Role of Fluids in Terrestrial and Extraterrestrial Processes; Springer Science & Business Media: Berlin, Germany, 2012; p. 806. [Google Scholar]
- Meinert, L.D. Skarns and skarn deposits. Geosci. Can. 1992, 19, 145–162. [Google Scholar]
- Ortoleva, P.; Merino, E.; Moore, C.; Chadam, J. Geochemical self-organization I; reaction-transport feedbacks and modeling approach. Am. J. Sci. 1987, 287, 979–1007. [Google Scholar] [CrossRef]















| Sample No. | Location | Description of Characteristics | Garnet Types |
|---|---|---|---|
| LS-024 | N46°28′14.82″; E90°6′3.98″ | Banded skarnization in volcanic rocks | Type 1 and Type 2a |
| LS-024-2 | N46°28′14.82″; E90°6′3.99″ | Banded skarnization in volcanic rocks | Type 1 and Type 2a |
| LS-024-3 | N46°28′14.82″; E90°6′3.100″ | Banded skarnization in volcanic rocks | Type 1 and Type 2a |
| TS-001 | N46°27′7.62″; E90°7′24.36″ | Garnet vein in volcanic rocks | Type 2b |
| TS-001-7 | N46°27′7.62″; E90°7′24.37” | Garnet vein in volcanic rocks | Type 2b |
| LS-026-4 | Stock heap | Massive magnetite ore with related epidote and disseminated pyrite | Type 2b |
| LS-006-4 | No.5 pit | Massive magnetite ore with taxitic pyrite | Type 3 |
| LS14-012-5 | Stock heap | Garnet in massive chalcopyrite ore | Type 3 |
| LS14-012-6 | Stock heap | Garnet in massive chalcopyrite ore | Type 3 |
| LS14-012-7 | Stock heap | Garnet in massive chalcopyrite ore | Type 3 |
| Samples | Spot No. | Types | Description | SiO2 | TiO2 | Al2O3 | FeOT | MnO | MgO | CaO | Na2O | Cr2O3 | Total | Si | Al iv | Al vi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS-024 | LS-024-GRT1 | Type 2a | slightly bright gray | 36.27 | 0.30 | 6.13 | 18.70 | 0.79 | 0.11 | 33.17 | 0.01 | b.d.l. | 95.49 | 3.06 | b.d.l. | 0.64 |
| LS-024 | LS-024-GRT3 | Type 1 | dark gray | 36.78 | 1.50 | 9.97 | 12.99 | 0.92 | 0.13 | 34.13 | 0.02 | 0.14 | 96.59 | 3.01 | b.d.l. | 0.99 |
| LS-024 | LS-024-GRT5 | Type 2a | slightly bright gray | 36.31 | 0.62 | 6.18 | 18.79 | 0.74 | 0.06 | 33.61 | 0.01 | 0.02 | 96.35 | 3.04 | b.d.l. | 0.64 |
| LS-024 | LS-024-GRT6 | Type 1 | dark gray | 36.90 | 0.87 | 9.68 | 14.34 | 0.99 | 0.16 | 33.64 | b.d.l. | 0.003 | 96.57 | 3.03 | b.d.l. | 0.97 |
| LS-024-2 | LS-024-2-1GRT | Type 1 | dark gray | 37.53 | 1.42 | 10.75 | 16.26 | 0.91 | 0.18 | 34.50 | 0.07 | 0.003 | 101.62 | 2.94 | 0.06 | 0.97 |
| LS-024-2 | LS-024-2-2GRT | Type 2a | slightly bright gray | 36.66 | 0.79 | 6.38 | 22.48 | 0.84 | 0.12 | 34.08 | 0.02 | 0.02 | 101.39 | 2.95 | 0.05 | 0.58 |
| LS-024-2 | LS-024-2-3GRT | Type 1 | dark gray | 37.38 | 0.23 | 10.73 | 17.20 | 0.87 | 0.10 | 34.28 | 0.02 | 0.37 | 101.18 | 2.95 | 0.05 | 0.99 |
| LS-024-2 | LS-024-2-4GRT | Type 2a | slightly bright gray | 36.77 | 0.69 | 6.60 | 22.62 | 0.96 | 0.13 | 33.59 | 0.01 | 0.08 | 101.45 | 2.96 | 0.04 | 0.61 |
| LS-024-2 | LS-024-2-5GRT | Type 1 | dark gray | 37.30 | 0.07 | 9.13 | 19.33 | 0.85 | 0.04 | 34.06 | 0.05 | 0.20 | 101.03 | 2.97 | 0.03 | 0.87 |
| LS-024-3 | LS-024-3-1 | Type 2a | slightly bright gray | 36.65 | 0.53 | 6.79 | 20.87 | 0.95 | 0.33 | 34.89 | b.d.l. | b.d.l. | 101.00 | 2.94 | 0.06 | 0.62 |
| LS-024-3 | LS-024-3-3 | Type 1 | dark gray | 36.85 | 1.35 | 10.71 | 14.69 | 1.06 | 0.20 | 36.05 | 0.06 | 0.02 | 100.99 | 2.91 | 0.09 | 0.94 |
| LS-024-3 | LS-024-3-5 | Type 1 | dark gray | 37.13 | 1.18 | 10.90 | 14.98 | 0.91 | 0.16 | 35.41 | 0.001 | 0.01 | 100.67 | 2.93 | 0.07 | 0.98 |
| LS-024-3 | LS-024-3-8 | Type 2a | slightly bright gray | 36.85 | 0.48 | 6.82 | 21.19 | 0.96 | 0.29 | 34.25 | 0.01 | b.d.l. | 100.86 | 2.96 | 0.04 | 0.64 |
| TS-001 | TS-001-10 | Type 2b | bright gray | 36.08 | 0.49 | 5.41 | 22.61 | 1.04 | b.d.l. | 33.29 | b.d.l. | b.d.l. | 98.92 | 2.98 | 0.02 | 0.54 |
| TS-001 | TS-001-11 | Type 2b | slightly bright gray | 36.62 | 0.16 | 6.93 | 19.67 | 0.34 | 0.18 | 35.43 | b.d.l. | b.d.l. | 99.34 | 2.98 | 0.02 | 0.67 |
| TS-001 | TS-001-20 | Type 2b | slightly bright gray | 36.29 | 0.78 | 5.78 | 21.77 | 0.89 | 0.25 | 34.07 | 0.05 | 0.01 | 99.88 | 2.96 | 0.04 | 0.54 |
| TS-001 | TS-001-GRT3 | Type 2b | slightly bright gray | 35.51 | 0.10 | 8.06 | 16.72 | 0.65 | 0.07 | 34.16 | 0.05 | 0.02 | 95.33 | 2.99 | 0.01 | 0.82 |
| TS-001 | TS-001-GRT4 | Type 2b | bright gray | 35.05 | 0.43 | 4.49 | 20.38 | 0.84 | 0.07 | 32.89 | 0.02 | 0.02 | 94.17 | 3.03 | b.d.l. | 0.48 |
| TS-001-7 | TS-001-7-1GRT | Type 2b | bright gray | 36.28 | 0.52 | 5.32 | 24.55 | 1.25 | 0.09 | 31.95 | 0.002 | b.d.l. | 99.94 | 2.98 | 0.02 | 0.52 |
| TS-001-7 | TS-001-7-2GRT | Type 2b | slightly bright gray | 35.85 | 0.44 | 5.36 | 24.00 | 0.85 | 0.09 | 33.38 | 0.01 | 0.01 | 99.99 | 2.94 | 0.06 | 0.49 |
| TS-001-7 | TS-001-7-3GRT | Type 2b | bright gray | 36.13 | 0.15 | 4.26 | 25.62 | 0.86 | 0.08 | 33.04 | 0.004 | b.d.l. | 100.16 | 2.97 | 0.03 | 0.41 |
| TS-001-7 | TS-001-7-4GRT | Type 2b | slightly bright gray | 36.08 | 0.49 | 5.49 | 23.91 | 0.79 | 0.10 | 33.21 | 0.01 | 0.003 | 100.10 | 2.96 | 0.04 | 0.51 |
| TS-001-7 | TS-001-7-5GRT | Type 2b | slightly bright gray | 36.08 | 0.73 | 6.06 | 23.10 | 1.04 | 0.09 | 32.99 | 0.03 | 0.01 | 100.18 | 2.95 | 0.05 | 0.56 |
| TS-001-7 | TS-001-7-6GRT | Type 2b | bright gray | 35.95 | 0.55 | 5.42 | 23.94 | 0.97 | 0.09 | 33.11 | b.d.l. | 0.001 | 100.06 | 2.95 | 0.05 | 0.50 |
| LS-026-4 | LS-026-4-G-2 | Type 2b | bright gray | 36.20 | 0.34 | 4.74 | 22.59 | 0.42 | 0.04 | 34.18 | 0.02 | b.d.l. | 98.53 | 3.00 | b.d.l. | 0.49 |
| LS-006-4 | LS-006-4-G-1 | Type 3 | homogeneity | 36.59 | 0.50 | 2.06 | 26.78 | 0.26 | 0.13 | 33.79 | 0.02 | b.d.l. | 100.14 | 3.02 | b.d.l. | 0.21 |
| LS14-012-5 | LS14-012-5-GRT1 | Type 3 | homogeneity | 35.69 | b.d.l. | 0.01 | 29.74 | 0.48 | 0.004 | 33.25 | b.d.l. | 0.01 | 99.18 | 3.02 | b.d.l. | 0.001 |
| LS14-012-5 | LS14-012-5-GRT2 | Type 3 | homogeneity | 35.79 | b.d.l. | 0.26 | 29.33 | 0.43 | b.d.l. | 33.40 | 0.02 | 0.02 | 99.25 | 3.02 | b.d.l. | 0.03 |
| LS14-012-5 | LS14-012-5-GRT3 | Type 3 | homogeneity | 36.20 | b.d.l. | 0.16 | 29.36 | 0.36 | b.d.l. | 33.74 | b.d.l. | b.d.l. | 99.82 | 3.03 | b.d.l. | 0.02 |
| LS14-012-5 | LS14-012-5-GRT-1 | Type 3 | homogeneity | 34.68 | b.d.l. | 0.09 | 28.94 | 0.43 | 0.01 | 33.28 | 0.01 | b.d.l. | 97.44 | 2.99 | 0.01 | b.d.l. |
| LS14-012-5 | LS14-012-5-GRT-2 | Type 3 | homogeneity | 34.83 | b.d.l. | 0.02 | 28.61 | 0.38 | 0.02 | 33.28 | 0.003 | b.d.l. | 97.14 | 3.00 | b.d.l. | 0.002 |
| LS14-012-5 | LS14-012-5-GRT-3 | Type 3 | homogeneity | 34.46 | b.d.l. | 0.01 | 28.94 | 0.39 | b.d.l. | 33.53 | b.d.l. | 0.01 | 97.33 | 2.97 | 0.001 | b.d.l. |
| LS14-012-6 | LS14-012-6-GRT1 | Type 3 | homogeneity | 35.92 | 0.01 | 1.45 | 27.89 | 0.39 | 0.02 | 33.72 | 0.02 | 0.001 | 99.41 | 3.01 | b.d.l. | 0.15 |
| LS14-012-6 | LS14-012-6-GRT2 | Type 3 | homogeneity | 35.59 | b.d.l. | 0.50 | 29.09 | 0.37 | b.d.l. | 33.33 | 0.01 | b.d.l. | 98.89 | 3.01 | b.d.l. | 0.05 |
| LS14-012-6 | LS14-012-6-GRT3 | Type 3 | homogeneity | 35.67 | b.d.l. | 0.37 | 29.64 | 0.46 | b.d.l. | 33.65 | 0.02 | 0.03 | 99.82 | 2.99 | 0.01 | 0.03 |
| LS14-012-6 | LS14-012-6-GRT-1 | Type 3 | homogeneity | 34.10 | b.d.l. | 0.16 | 28.87 | 0.41 | b.d.l. | 33.13 | 0.02 | 0.01 | 96.69 | 2.96 | 0.02 | b.d.l. |
| LS14-012-7 | LS14-012-7-GRT-1 | Type 3 | homogeneity | 34.55 | b.d.l. | 0.004 | 28.99 | 0.38 | 0.01 | 33.28 | 0.02 | 0.02 | 97.26 | 2.98 | 0.0004 | b.d.l. |
| Samples | Spot No. | Types | Description | Ti | Cr | Fe3+ | Fe2+ | Mn | Mg | Ca | Alm | Adr | Grs | Prp | Sps | Uv |
| LS-024 | LS-024-GRT1 | Type 2a | slightly bright gray | 0.02 | b.d.l. | 1.09 | 0.23 | 0.06 | 0.01 | 3.00 | 0.00 | 64.09 | 33.12 | 0.56 | 2.23 | 0.00 |
| LS-024 | LS-024-GRT3 | Type 1 | dark gray | 0.09 | 0.01 | 0.77 | 0.12 | 0.06 | 0.02 | 2.99 | 0.00 | 44.22 | 52.21 | 0.60 | 2.45 | 0.52 |
| LS-024 | LS-024-GRT5 | Type 2a | slightly bright gray | 0.04 | 0.001 | 1.09 | 0.23 | 0.05 | 0.01 | 3.01 | 0.00 | 63.99 | 33.58 | 0.29 | 2.05 | 0.09 |
| LS-024 | LS-024-GRT6 | Type 1 | dark gray | 0.05 | 0.0002 | 0.81 | 0.17 | 0.07 | 0.02 | 2.96 | 0.00 | 46.52 | 50.10 | 0.75 | 2.62 | 0.01 |
| LS-024-2 | LS-024-2-1GRT | Type 1 | dark gray | 0.08 | 0.0002 | 0.81 | 0.26 | 0.06 | 0.02 | 2.90 | 0.00 | 44.87 | 52.14 | 0.76 | 2.22 | 0.01 |
| LS-024-2 | LS-024-2-2GRT | Type 2a | slightly bright gray | 0.05 | 0.001 | 1.16 | 0.36 | 0.06 | 0.01 | 2.94 | 0.00 | 65.60 | 31.62 | 0.53 | 2.17 | 0.08 |
| LS-024-2 | LS-024-2-3GRT | Type 1 | dark gray | 0.01 | 0.02 | 0.84 | 0.30 | 0.06 | 0.01 | 2.90 | 0.00 | 44.95 | 51.30 | 0.42 | 2.10 | 1.24 |
| LS-024-2 | LS-024-2-4GRT | Type 2a | slightly bright gray | 0.04 | 0.01 | 1.14 | 0.38 | 0.07 | 0.01 | 2.89 | 0.00 | 64.34 | 32.33 | 0.57 | 2.47 | 0.29 |
| LS-024-2 | LS-024-2-5GRT | Type 1 | dark gray | 0.004 | 0.01 | 0.95 | 0.34 | 0.06 | 0.005 | 2.91 | 0.00 | 52.24 | 44.80 | 0.18 | 2.09 | 0.68 |
| LS-024-3 | LS-024-3-1 | Type 2a | slightly bright gray | 0.03 | b.d.l. | 1.14 | 0.26 | 0.06 | 0.04 | 3.00 | 0.00 | 63.99 | 32.12 | 1.48 | 2.42 | 0.00 |
| LS-024-3 | LS-024-3-3 | Type 1 | dark gray | 0.08 | 0.001 | 0.84 | 0.13 | 0.07 | 0.02 | 3.05 | 0.00 | 45.76 | 50.77 | 0.85 | 2.56 | 0.05 |
| LS-024-3 | LS-024-3-5 | Type 1 | dark gray | 0.07 | 0.0003 | 0.81 | 0.18 | 0.06 | 0.02 | 3.00 | 0.00 | 44.48 | 52.61 | 0.68 | 2.21 | 0.02 |
| LS-024-3 | LS-024-3-8 | Type 2a | slightly bright gray | 0.03 | b.d.l. | 1.12 | 0.30 | 0.07 | 0.03 | 2.95 | 0.00 | 63.48 | 32.75 | 1.30 | 2.47 | 0.00 |
| TS-001 | TS-001-10 | Type 2b | bright gray | 0.03 | b.d.l. | 1.21 | 0.35 | 0.07 | b.d.l. | 2.95 | 0.00 | 69.65 | 27.55 | 0.00 | 2.80 | 0.00 |
| TS-001 | TS-001-11 | Type 2b | slightly bright gray | 0.01 | b.d.l. | 1.12 | 0.22 | 0.02 | 0.02 | 3.09 | 0.00 | 62.69 | 35.59 | 0.83 | 0.89 | 0.00 |
| TS-001 | TS-001-20 | Type 2b | slightly bright gray | 0.05 | 0.001 | 1.19 | 0.30 | 0.06 | 0.03 | 2.98 | 0.00 | 68.11 | 28.35 | 1.16 | 2.36 | 0.03 |
| TS-001 | TS-001-GRT3 | Type 2b | slightly bright gray | 0.01 | 0.001 | 1.00 | 0.18 | 0.05 | 0.01 | 3.08 | 0.00 | 55.47 | 42.42 | 0.32 | 1.72 | 0.07 |
| TS-001 | TS-001-GRT4 | Type 2b | bright gray | 0.03 | 0.001 | 1.23 | 0.24 | 0.06 | 0.01 | 3.04 | 0.00 | 72.86 | 24.27 | 0.37 | 2.42 | 0.08 |
| TS-001-7 | TS-001-7-1GRT | Type 2b | bright gray | 0.03 | b.d.l. | 1.22 | 0.47 | 0.09 | 0.01 | 2.81 | 0.00 | 70.32 | 25.94 | 0.40 | 3.33 | 0.00 |
| TS-001-7 | TS-001-7-2GRT | Type 2b | slightly bright gray | 0.03 | 0.001 | 1.25 | 0.40 | 0.06 | 0.01 | 2.94 | 0.00 | 70.65 | 26.64 | 0.43 | 2.23 | 0.05 |
| TS-001-7 | TS-001-7-3GRT | Type 2b | bright gray | 0.01 | b.d.l. | 1.33 | 0.44 | 0.06 | 0.01 | 2.91 | 0.00 | 76.22 | 21.10 | 0.40 | 2.29 | 0.00 |
| TS-001-7 | TS-001-7-4GRT | Type 2b | slightly bright gray | 0.03 | 0.0002 | 1.23 | 0.41 | 0.05 | 0.01 | 2.91 | 0.00 | 69.87 | 27.57 | 0.47 | 2.08 | 0.01 |
| TS-001-7 | TS-001-7-5GRT | Type 2b | slightly bright gray | 0.04 | 0.001 | 1.18 | 0.40 | 0.07 | 0.01 | 2.89 | 0.00 | 66.83 | 29.97 | 0.42 | 2.73 | 0.05 |
| TS-001-7 | TS-001-7-6GRT | Type 2b | bright gray | 0.03 | 0.0001 | 1.24 | 0.41 | 0.07 | 0.01 | 2.91 | 0.00 | 70.22 | 26.78 | 0.44 | 2.56 | 0.00 |
| LS-026-4 | LS-026-4-G-2 | Type 2b | bright gray | 0.02 | b.d.l. | 1.25 | 0.31 | 0.03 | 0.01 | 3.04 | 0.00 | 73.04 | 25.61 | 0.21 | 1.14 | 0.00 |
| LS-006-4 | LS-006-4-G-1 | Type 3 | homogeneity | 0.03 | b.d.l. | 1.45 | 0.40 | 0.02 | 0.02 | 2.99 | 0.00 | 87.83 | 10.79 | 0.64 | 0.74 | 0.00 |
| LS14-012-5 | LS14-012-5-GRT1 | Type 3 | homogeneity | b.d.l. | 0.0005 | 1.64 | 0.46 | 0.03 | 0.001 | 3.01 | 0.00 | 99.90 | 0.00 | 0.02 | 0.05 | 0.03 |
| LS14-012-5 | LS14-012-5-GRT2 | Type 3 | homogeneity | b.d.l. | 0.001 | 1.62 | 0.45 | 0.03 | b.d.l. | 3.02 | 0.00 | 98.32 | 0.36 | 0.00 | 1.23 | 0.09 |
| LS14-012-5 | LS14-012-5-GRT3 | Type 3 | homogeneity | b.d.l. | b.d.l. | 1.62 | 0.44 | 0.03 | b.d.l. | 3.03 | 0.00 | 99.02 | 0.00 | 0.00 | 0.98 | 0.00 |
| LS14-012-5 | LS14-012-5-GRT-1 | Type 3 | homogeneity | b.d.l. | b.d.l. | 1.66 | 0.42 | 0.03 | 0.001 | 3.07 | 0.00 | 99.46 | 0.00 | 0.03 | 0.51 | 0.00 |
| LS14-012-5 | LS14-012-5-GRT-2 | Type 3 | homogeneity | b.d.l. | b.d.l. | 1.65 | 0.41 | 0.03 | 0.002 | 3.07 | 0.00 | 99.91 | 0.00 | 0.09 | 0.00 | 0.00 |
| LS14-012-5 | LS14-012-5-GRT-3 | Type 3 | homogeneity | b.d.l. | 0.0004 | 1.68 | 0.41 | 0.03 | b.d.l. | 3.10 | 0.00 | 99.91 | 0.00 | 0.00 | 0.07 | 0.02 |
| LS14-012-6 | LS14-012-6-GRT1 | Type 3 | homogeneity | 0.001 | 0.0001 | 1.53 | 0.42 | 0.03 | 0.002 | 3.02 | 0.00 | 91.47 | 7.36 | 0.07 | 1.09 | 0.00 |
| LS14-012-6 | LS14-012-6-GRT2 | Type 3 | homogeneity | b.d.l. | b.d.l. | 1.61 | 0.45 | 0.03 | b.d.l. | 3.02 | 0.00 | 96.98 | 1.95 | 0.00 | 1.07 | 0.00 |
| LS14-012-6 | LS14-012-6-GRT3 | Type 3 | homogeneity | b.d.l. | 0.002 | 1.63 | 0.45 | 0.03 | b.d.l. | 3.03 | 0.00 | 97.70 | 0.90 | 0.00 | 1.29 | 0.11 |
| LS14-012-6 | LS14-012-6-GRT-1 | Type 3 | homogeneity | b.d.l. | 0.001 | 1.67 | 0.43 | 0.03 | b.d.l. | 3.08 | 0.00 | 99.01 | 0.00 | 0.00 | 0.94 | 0.05 |
| LS14-012-7 | LS14-012-7-GRT-1 | Type 3 | homogeneity | b.d.l. | 0.001 | 1.67 | 0.42 | 0.03 | 0.001 | 3.08 | 0.00 | 99.91 | 0.00 | 0.02 | 0.00 | 0.07 |
| Samples | Spot No. | Types | Calibrated EPMA Spot No. | SiO2 | TiO2 | Al2O3 | FeOT | MnO | MgO | CaO | Na2O | K2O | P2O5 | Sc | V | Cr | Co | Ni | Cu | Zn | Ga |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | wt.% | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ||||
| LS-024 | LS-024-GRT1 | Type 2a | LS-024-GRT1 | 36.2 | 0.39 | 8.89 | 19.5 | 0.95 | 0.11 | 34.4 | 0.005 | b.d.l. | 0.01 | 7.90 | 676 | 79.7 | 0.62 | 0.28 | b.d.l. | 3.59 | 14.1 |
| LS-024 | LS-024-GRT2 | Type 1 | LS-024-3-3 | 36.7 | 1.96 | 9.84 | 17.4 | 0.90 | 0.20 | 35.7 | 0.01 | 0.001 | 0.01 | 10.6 | 1138 | 44.5 | 0.76 | 0.77 | 1.52 | 2.77 | 21.6 |
| LS-024 | LS-024-GRT3 | Type 1 | LS-024-GRT3 | 36.7 | 1.88 | 10.7 | 15.3 | 1.13 | 0.27 | 35.1 | 0.02 | 0.02 | 0.01 | 33.4 | 1531 | 726 | 0.78 | 0.85 | 7.17 | 5.17 | 11.3 |
| LS-024 | LS-024-GRT4 | Type 1 | LS-024-3-5 | 36.7 | 0.72 | 8.64 | 19.0 | 0.88 | 0.12 | 34.8 | 0.01 | 0.003 | 0.01 | 23.7 | 714 | 55.7 | 0.47 | 0.46 | 88.5 | 3.11 | 14.7 |
| LS-024 | LS-024-GRT5 | Type 2a | LS-024-GRT5 | 36.3 | 0.76 | 7.82 | 20.5 | 0.83 | 0.10 | 34.0 | 0.004 | 0.0005 | 0.01 | 4.37 | 518 | 10.6 | 1.59 | 42.5 | 414 | 51.5 | 18.3 |
| LS-024 | LS-024-GRT6 | Type 1 | LS-024-GRT6 | 36.8 | 0.91 | 9.88 | 17.5 | 1.12 | 0.13 | 34.3 | 0.01 | 0.001 | 0.01 | 3.61 | 412 | 41.1 | 0.49 | 0.50 | 77.1 | 2.21 | 10.8 |
| TS-001 | TS-001-GRT1 | Type 2b | TS-001-10 | 36.0 | 0.50 | 5.77 | 23.6 | 1.10 | 0.10 | 34.0 | 0.003 | b.d.l. | 0.01 | 8.60 | 414 | 12.2 | 1.65 | 0.97 | b.d.l. | 3.71 | 11.4 |
| TS-001 | TS-001-GRT2 | Type 2b | TS-001-11 | 36.0 | 0.93 | 5.66 | 23.5 | 1.04 | 0.12 | 34.1 | 0.01 | 0.004 | 0.01 | 27.8 | 813 | 18.1 | 1.61 | 1.53 | 1.57 | 7.45 | 11.4 |
| TS-001 | TS-001-GRT3 | Type 2b | TS-001-GRT3 | 36.0 | 0.49 | 7.64 | 20.4 | 1.03 | 0.12 | 33.5 | 0.01 | 0.001 | 0.01 | 6.73 | 312 | 1.93 | 1.44 | 1.05 | b.d.l. | 2.85 | 11.4 |
| TS-001 | TS-001-GRT4 | Type 2b | TS-001-GRT4 | 36.0 | 0.54 | 5.89 | 23.2 | 1.12 | 0.11 | 33.0 | 0.01 | 0.003 | 0.01 | 6.67 | 440 | 23.5 | 1.77 | 1.25 | 3.72 | 4.45 | 12.7 |
| TS-001 | TS-001-GRT5 | Type 2b | TS-001-20 | 36.3 | 0.63 | 5.34 | 20.8 | 0.85 | 1.94 | 30.4 | 0.02 | 0.01 | 0.01 | 21.0 | 325 | 4.97 | 53.8 | 16.4 | 121 | 37.2 | 12.3 |
| LS14-012-5 | LS14-012-GRT1 | Type 3 | LS14-012-5-GRT-1 | 35.7 | 0.001 | 0.14 | 30.0 | 0.45 | 0.02 | 34.8 | 0.002 | b.d.l. | 0.01 | 0.74 | 12.3 | b.d.l. | 0.25 | 0.66 | 1.11 | 1.91 | 4.36 |
| LS14-012-5 | LS14-012-GRT2 | Type 3 | LS14-012-5-GRT-2 | 36.1 | 0.0003 | 0.04 | 30.7 | 0.46 | 0.02 | 35.7 | 0.002 | b.d.l. | 0.01 | 0.79 | 3.61 | b.d.l. | 0.24 | 1.19 | b.d.l. | 0.92 | 2.90 |
| LS14-012-5 | LS14-012-GRT3 | Type 3 | LS14-012-5-GRT-3 | 35.6 | 0.001 | 0.04 | 30.4 | 0.48 | 0.01 | 34.9 | 0.002 | b.d.l. | 0.01 | 0.77 | 8.96 | b.d.l. | 0.22 | 0.64 | b.d.l. | 2.17 | 2.44 |
| LS14-012-6 | LS14-012-GRT4 | Type 3 | LS14-012-6-GRT-1 | 35.5 | 0.005 | 0.25 | 29.8 | 0.43 | 0.02 | 33.7 | 0.003 | b.d.l. | 0.01 | 0.64 | 145 | b.d.l. | 0.51 | 0.74 | b.d.l. | 3.25 | 12.5 |
| LS14-012-7 | LS14-012-GRT5 | Type 3 | LS14-012-7-GRT-1 | 35.6 | 0.0001 | 0.06 | 30.2 | 0.46 | 0.03 | 34.5 | 0.004 | 0.0005 | 0.01 | 1.01 | 22.4 | 1.65 | 0.27 | 0.92 | 9.34 | 3.06 | 6.56 |
| Samples | Spot No. | Types | Calibrated EPMA Spot no. | Ge | As | Rb | Sr | Y | Zr | Nb | Sn | Cs | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb |
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ||||
| LS-024 | LS-024-GRT1 | Type 2a | LS-024-GRT1 | 4.09 | 11.7 | 0.09 | 0.95 | 23.2 | 17.0 | 0.24 | 6.65 | b.d.l. | 1.16 | b.d.l. | 0.28 | 0.16 | 3.06 | 5.74 | 6.59 | 6.79 | 0.90 |
| LS-024 | LS-024-GRT2 | Type 1 | LS-024-3-3 | 5.04 | 13.6 | b.d.l. | 0.48 | 20.0 | 32.8 | 3.41 | 4.56 | 0.03 | 0.35 | b.d.l. | 0.16 | 0.12 | 2.29 | 4.47 | 8.32 | 7.55 | 1.09 |
| LS-024 | LS-024-GRT3 | Type 1 | LS-024-GRT3 | 3.70 | 11.2 | 0.14 | 0.99 | 27.9 | 47.2 | 2.15 | 1.17 | b.d.l. | 1.56 | 0.04 | 0.10 | 0.02 | 0.35 | 0.42 | 0.36 | 1.32 | 0.40 |
| LS-024 | LS-024-GRT4 | Type 1 | LS-024-3-5 | 3.98 | 13.7 | 0.12 | 1.16 | 18.2 | 26.2 | 0.42 | 4.59 | 0.06 | 1.36 | 0.03 | 0.12 | 0.04 | 0.88 | 1.41 | 3.41 | 2.66 | 0.46 |
| LS-024 | LS-024-GRT5 | Type 2a | LS-024-GRT5 | 3.99 | 47.1 | b.d.l. | 0.92 | 11.7 | 19.3 | 0.26 | 6.14 | b.d.l. | 0.31 | 0.37 | 0.99 | 0.23 | 4.05 | 5.40 | 6.80 | 5.79 | 0.59 |
| LS-024 | LS-024-GRT6 | Type 1 | LS-024-GRT6 | 3.70 | 14.0 | 0.07 | 0.55 | 10.0 | 21.0 | 2.33 | 4.98 | b.d.l. | 0.32 | 0.03 | 0.04 | 0.02 | 0.13 | 0.19 | 1.90 | 1.14 | 0.25 |
| TS-001 | TS-001-GRT1 | Type 2b | TS-001-10 | 11.8 | 19.8 | b.d.l. | 0.25 | 31.0 | 76.7 | 0.80 | 11.2 | b.d.l. | b.d.l. | 0.15 | 2.79 | 1.34 | 14.3 | 6.56 | 8.66 | 6.60 | 0.95 |
| TS-001 | TS-001-GRT2 | Type 2b | TS-001-11 | 19.2 | 18.5 | 0.36 | 1.49 | 91.1 | 131 | 0.91 | 7.42 | 0.23 | 0.89 | 0.18 | 2.79 | 1.27 | 18.2 | 18.2 | 14.7 | 25.1 | 3.35 |
| TS-001 | TS-001-GRT3 | Type 2b | TS-001-GRT3 | 8.61 | 18.9 | 0.06 | 0.49 | 31.2 | 51.3 | 1.36 | 5.67 | 0.04 | b.d.l. | 0.11 | 1.88 | 0.84 | 10.2 | 6.12 | 7.62 | 6.86 | 1.00 |
| TS-001 | TS-001-GRT4 | Type 2b | TS-001-GRT4 | 10.9 | 23.8 | 0.17 | 0.81 | 28.5 | 76.9 | 1.07 | 9.29 | b.d.l. | 0.49 | 0.19 | 2.27 | 1.03 | 13.3 | 7.37 | 9.04 | 8.01 | 0.91 |
| TS-001 | TS-001-GRT5 | Type 2b | TS-001-20 | 6.07 | 29.9 | 0.10 | 3.19 | 18.0 | 141 | 0.47 | 8.57 | b.d.l. | 9.68 | 0.36 | 7.11 | 1.28 | 14.0 | 5.84 | 7.99 | 4.44 | 0.46 |
| LS14-012-5 | LS14-012-GRT1 | Type 3 | LS14-012-5-GRT-1 | 1.08 | 1810 | b.d.l. | 1.01 | 0.51 | b.d.l. | b.d.l. | 8.63 | b.d.l. | b.d.l. | 1.55 | 0.80 | 0.06 | 0.21 | 0.09 | 0.23 | b.d.l. | 0.01 |
| LS14-012-5 | LS14-012-GRT2 | Type 3 | LS14-012-5-GRT-2 | 1.06 | 1462 | b.d.l. | 1.02 | 0.36 | b.d.l. | b.d.l. | 5.48 | 0.07 | b.d.l. | 0.63 | 0.41 | 0.05 | 0.22 | 0.04 | 0.21 | b.d.l. | 0.01 |
| LS14-012-5 | LS14-012-GRT3 | Type 3 | LS14-012-5-GRT-3 | b.d.l. | 2016 | b.d.l. | 0.95 | 0.66 | b.d.l. | b.d.l. | 4.67 | b.d.l. | b.d.l. | 0.61 | 0.57 | 0.04 | 0.31 | 0.12 | 0.32 | 0.06 | 0.01 |
| LS14-012-6 | LS14-012-GRT4 | Type 3 | LS14-012-6-GRT-1 | 1.17 | 3245 | b.d.l. | 0.72 | 0.62 | 0.05 | 0.05 | 14.1 | b.d.l. | b.d.l. | 2.95 | 4.37 | 0.37 | 0.96 | 0.13 | 2.25 | 0.17 | 0.03 |
| LS14-012-7 | LS14-012-GRT5 | Type 3 | LS14-012-7-GRT-1 | 1.33 | 2332 | b.d.l. | 0.80 | 37.1 | b.d.l. | b.d.l. | 2.35 | b.d.l. | b.d.l. | 20.0 | 28.0 | 2.88 | 14.2 | 4.05 | 5.46 | 6.26 | 0.83 |
| Samples | Spot No. | Types | Calibrated EPMA Spot No. | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | Au | Bi | Th | U | ∑REE | ∑LREE/ ∑HREE | (La/Yb)N | δEu | δCe | |
| ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | ppm | |||||||||
| LS-024 | LS-024-GRT1 | Type 2a | LS-024-GRT1 | 5.16 | 0.85 | 1.92 | 0.23 | 1.45 | 0.22 | 0.38 | 0.04 | 0.01 | b.d.l. | 0.07 | 1.38 | 33.4 | 0.90 | b.d.l. | 3.22 | b.d.l. | |
| LS-024 | LS-024-GRT2 | Type 1 | LS-024-3-3 | 5.04 | 0.72 | 1.64 | 0.18 | 1.09 | 0.11 | 0.62 | 0.12 | b.d.l. | b.d.l. | 0.89 | 9.32 | 32.8 | 0.88 | b.d.l. | 4.35 | b.d.l. | |
| LS-024 | LS-024-GRT3 | Type 1 | LS-024-GRT3 | 3.72 | 0.97 | 3.44 | 0.62 | 4.13 | 0.61 | 1.30 | 0.06 | 0.01 | b.d.l. | 0.09 | 0.41 | 16.5 | 0.09 | 0.01 | 1.36 | 0.85 | |
| LS-024 | LS-024-GRT4 | Type 1 | LS-024-3-5 | 3.40 | 0.70 | 1.72 | 0.26 | 1.56 | 0.21 | 0.58 | 0.02 | b.d.l. | b.d.l. | 0.16 | 2.23 | 16.8 | 0.54 | 0.02 | 5.29 | 0.65 | |
| LS-024 | LS-024-GRT5 | Type 2a | LS-024-GRT5 | 2.41 | 0.46 | 1.08 | 0.15 | 0.73 | 0.13 | 0.33 | 0.03 | b.d.l. | b.d.l. | 0.09 | 2.64 | 29.2 | 1.57 | 0.37 | 3.69 | 0.82 | |
| LS-024 | LS-024-GRT6 | Type 1 | LS-024-GRT6 | 1.94 | 0.38 | 1.09 | 0.14 | 0.76 | 0.12 | 0.48 | 0.15 | b.d.l. | b.d.l. | 0.03 | 2.07 | 8.14 | 0.40 | 0.02 | 9.61 | 0.46 | |
| TS-001 | TS-001-GRT1 | Type 2b | TS-001-10 | 5.79 | 1.06 | 2.94 | 0.46 | 2.76 | 0.41 | 1.74 | 0.06 | b.d.l. | 0.03 | 0.21 | 5.08 | 54.8 | 1.61 | 0.04 | 3.98 | 0.62 | |
| TS-001 | TS-001-GRT2 | Type 2b | TS-001-11 | 18.7 | 3.34 | 8.57 | 1.24 | 7.36 | 1.11 | 4.34 | 0.25 | b.d.l. | 0.03 | 0.35 | 2.82 | 124 | 0.81 | 0.02 | 2.11 | 0.64 | |
| TS-001 | TS-001-GRT3 | Type 2b | TS-001-GRT3 | 6.34 | 1.13 | 3.21 | 0.43 | 3.00 | 0.37 | 1.39 | 0.15 | b.d.l. | b.d.l. | 0.32 | 4.97 | 49.1 | 1.20 | 0.03 | 3.58 | 0.66 | |
| TS-001 | TS-001-GRT4 | Type 2b | TS-001-GRT4 | 5.60 | 1.08 | 2.95 | 0.42 | 2.80 | 0.35 | 1.73 | 0.09 | 0.03 | 0.04 | 0.28 | 4.89 | 55.3 | 1.50 | 0.05 | 3.58 | 0.64 | |
| TS-001 | TS-001-GRT5 | Type 2b | TS-001-20 | 3.29 | 0.63 | 1.68 | 0.25 | 1.95 | 0.27 | 4.42 | 0.07 | b.d.l. | 0.03 | 0.47 | 5.58 | 49.6 | 2.82 | 0.13 | 4.61 | 1.55 | |
| LS14-012-5 | LS14-012-GRT1 | Type 3 | LS14-012-5-GRT-1 | 0.07 | b.d.l. | 0.03 | b.d.l. | 0.04 | 0.01 | b.d.l. | b.d.l. | 0.02 | 0.09 | b.d.l. | 0.10 | 3.10 | 17.9 | 26.7 | b.d.l. | 0.37 | |
| LS14-012-5 | LS14-012-GRT2 | Type 3 | LS14-012-5-GRT-2 | 0.05 | 0.01 | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.02 | 0.09 | b.d.l. | 0.01 | 1.66 | 13.7 | b.d.l. | b.d.l. | 0.42 | |
| LS14-012-5 | LS14-012-GRT3 | Type 3 | LS14-012-5-GRT-3 | 0.09 | 0.02 | 0.06 | b.d.l. | 0.04 | 0.01 | b.d.l. | b.d.l. | b.d.l. | 0.07 | b.d.l. | 0.02 | 2.26 | 6.91 | 11.0 | 10.6 | 0.62 | |
| LS14-012-6 | LS14-012-GRT4 | Type 3 | LS14-012-6-GRT-1 | 0.10 | 0.02 | 0.05 | 0.01 | 0.03 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 0.12 | b.d.l. | 1.77 | 11.4 | 26.5 | 61.9 | 45.5 | 0.87 | |
| LS14-012-7 | LS14-012-GRT5 | Type 3 | LS14-012-7-GRT-1 | 4.97 | 0.88 | 1.98 | 0.21 | 1.23 | 0.14 | 0.004 | b.d.l. | 0.01 | 0.10 | 0.01 | 0.23 | 91.1 | 4.53 | 11.6 | 3.31 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, P.; Zhang, Y.; Xie, Y. Chemical Composition and Genesis Implication of Garnet from the Laoshankou Fe-Cu-Au Deposit, the Northern Margin of East Junggar, NW China. Minerals 2021, 11, 334. https://0-doi-org.brum.beds.ac.uk/10.3390/min11030334
Liang P, Zhang Y, Xie Y. Chemical Composition and Genesis Implication of Garnet from the Laoshankou Fe-Cu-Au Deposit, the Northern Margin of East Junggar, NW China. Minerals. 2021; 11(3):334. https://0-doi-org.brum.beds.ac.uk/10.3390/min11030334
Chicago/Turabian StyleLiang, Pei, Yu Zhang, and Yuling Xie. 2021. "Chemical Composition and Genesis Implication of Garnet from the Laoshankou Fe-Cu-Au Deposit, the Northern Margin of East Junggar, NW China" Minerals 11, no. 3: 334. https://0-doi-org.brum.beds.ac.uk/10.3390/min11030334