1. Introduction
The first uranium mineral deposits in the Sierra Peña Blanca range, Chihuahua, were localized and characterized in the 1960s by the National Nuclear Energy Commission (CNEN) of Mexico. Later, sinkholes and mining pits were opened during mining and exploration activities led by URAMEX (“Uranio Mexicano”). Potassium (carnotite), calcium (metatyuyamunite) and cesium (margaritasite) uranyl vanadates were identified in the Margaritas deposit. These activities concerning the mineral deposits were suspended in 1983 [
1]. The uranium ore that was extracted and stored outside has been exposed to weathering. Since surface waters in desert floodplains can transport uranium into the environment, the minerals still present in Peña Blanca are of interest for public health reasons.
In a previous work, the synthesis of the uranyl vanadates of potassium and cesium was reported and their crystalline structures were proposed [
2]. The synthesis methods were based on the mild hydrothermal reactions of uranyl pyrovanadate with alkali metal hydroxides [
3,
4,
5].
In this context, at the beginning of the 1980s, a mineral compound based on cesium uranyl vanadate was collected from the Margaritas deposit. It was named “margaritasite”. In 1982, Wenrich et al. [
6] published a work on this newly discovered mineral. It was singled out and studied for the first time by various characterization methods. An interesting experiment was carried out that led to researchers obtaining margaritasite from carnotite under controlled laboratory conditions. Analytical techniques included X-ray powder diffraction and scanning electron microscopy. Diffraction experiments in [
6] demonstrate that, in the samples studied in that research, both margaritasite and carnotite appear quite close to model stoichiometric phases. These authors did not find a disordered solid solution of K and Cs. A question that [
6] left open is whether, under field weathering conditions, various margaritasite–carnotite solid solutions occur. To the best of our knowledge, no model has been proposed to justify the presence of cesium in the mine environment.
In this study, we conducted a comprehensive geological and crystallographic characterization of the natural margaritasite–carnotite-type mineral. To achieve this, we employed state-of-the-art chemical–structural techniques, including high-resolution X-ray diffraction (XRD), scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDX), selected area electron diffraction (SAED), and X-ray absorption spectroscopy (XAS). These techniques were correlated to obtain a detailed and comprehensive information level on the minerals studied. Furthermore, we combined the results of the crystallographic analysis with a geological description of the uranium region and the specific Margaritas deposit. This allowed us to propose the origin of cesium and a formation model of this rare mineral, thereby contributing to the understanding of the geological and crystallographic aspects of the Margaritas deposit.
2. Uranyl Vanadates Crystallography
Burns et al. [
7,
8,
9] proposed a structural hierarchy for uranyl compounds, built upon the types of bipyramids that UO
2+2 forms with the equatorial oxygens and on the connectivity of U ions with other anionic and cationic environments. The uranyl vanadates carnotite [K
2(UO
2)
2(VO
4)
2·nH
2O] and margaritasite [Cs
2(UO
2)
2(VO
4)
2·nH
2O] are described within the sheet anion topologies containing triangles, squares, and pentagons, and more specifically, within the francevillite-type anion topology [
8,
9]. The scheme of the carnotite and margaritasite structure is shown in
Figure 1. It consists of two UO
7 pentagonal bipyramids, with a uranyl UO
2 axis, joined at one edge, which in turn are joined to another pair by a vertex and have contact with vanadium tetrahedra on their respective edges. Since the 1950s, various works dedicated to the synthesis [
2,
10,
11], crystallographic characterization [
12,
13], and thermodynamics [
3,
14] of these uranyl vanadates have been published.
The space group of margaritasite/carnotite is number 14, according to the International Union of Crystallography classification. It corresponds to the monoclinic system. Depending on the coordinate system settings, this group may be represented by different notations. Earlier reports on margaritasite/carnotite considered alternative axis settings and applied the P 1 2
1/a 1 notation for the same space group. In this paper, we follow the convention of the Bilbao Crystallographic Server [
15] and thus denote this symmetry group with the symbols P 1 2
1/c 1.
3. Geology of the Uranium Region Sierra Peña Blanca, Chihuahua
The Sierra de Peña Blanca (SPB) is a fault-block mountain range located 70 km northeast of Chihuahua city, with UTM coordinates of 395,508 east and 3,223,656 north, in zone 13R. The mountain range is about 100 km long in a north–south direction, with a width of up to 15 km, and presents many faults and fractures, resulting in an irregular and abrupt topography. Tectonic activities there have led to the formation of an ignimbrite pile, which was significantly enriched with uranium mineralization. The Peña Blanca uranium district hosts about 40% of the Mexican uranium reserves and consequently has garnered much interest for its geological–mineralogical characteristics. The uranium deposits are stratigraphically controlled in these volcanic rocks, mainly rhyolitic tuffs, and in the underlying Cretaceous rocks, with or without additional mineralization processes. Some of these main ores and their specific mineralizations are known as Nopal I, Puerto III, Domitila, and Margaritas [
16].
The Nopal I ore deposit, in particular, has been widely studied, with its genesis, mineralogy and geology described by, to name a few, Goodell et al. [
17,
18], George-Aniel et al. [
19], Angiboust et al. [
20], and others [
21,
22,
23,
24,
25]. Indeed, it has been a natural representative of a uranium ore repository in natural equilibrium with its environment for thousands of years, if not more.
The geological basement of SPB (
Figure 2) consists of three lithological units, identified as Cretaceous sedimentary, Cuervo, and Pozos formations, with an irregular and localized presence of tertiary volcanic rocks. Within those rocks are found the formations that contain uranium deposits and, thus, mining possibilities, such as in the Nopal, Escuadra, and Chontes Formations [
21].
The Cretaceous sedimentary rocks are formed mainly by sandstones and siltstones, and include conglomerates of calcareous reef formations, with fossil and chert (Buda Formation). It extends out to the south and northeast of the uranium district and dates from the Albian–Cenomanian ages. The Cuervo formation rests unconformably on the Edwards Formation. In the uranium district it consists of two ignimbrites interspersed with volcanoclastic and sedimentary deposits (conglomerates). It is estimated that it belongs to the Upper Cretaceous–Paleogene, because it is folded together with the sedimentary Cretaceous [
20,
21]. The Pozos Formation is deposited unconformably above the Cuervo Formation. It is composed of polymictic conglomerates of volcanic fragments, limestone, and chert, with intercalations of sandstone lenses, as well as thin layers of tuff (pumice) [
25].
The Nopal formation can be described lithologically as a crystal-rich, rhyolitic ash flow tuff with quartz and sanidine phenocrysts, displaying a devitrified matrix in addition to unaltered biotite crystals [
21] and presenting highly altered basal vitrophyres [
25]. The Nopal Formation has been dated to 43.8 M.a. [
16] and is unconformably superimposed onto the Coloradas formation (lithic tuff), which in turn unconformably overlies the Pozos Formation. As for the Chontes Formation, it is a basal red breccio-conglomerate with red sandstone lenses [
21] on which the Escuadra formation was deposited in a discordant manner. Some authors consider that it was formed later than the Escuadra Formation due to the abrupt changes in its thickness [
21,
26]. Finally, the Escuadra Formation is a pinkish crystalline ignimbrite, with altered and leached areas, which contains quartz and sanidine phenocrysts (iridescent). It has been dated to 38 M.a. [
21].
The diagenesis of uranium ores (
Figure 3) in the Peña Blanca district has been discussed by various authors, for example [
18,
19,
23]. The uranium primarily originated from peralkaline rocks (with U ≈ 20 ppm) [
18] issued from the Nido blocks (Sierra de la Campana), which were found approximately between 30 to 40 km west of the current Peña Blanca uranium district, thus ex situ. Ref. [
18] suggested that the migration of uranium (~35 M.a.) from the del Nido Block to Peña Blanca resulted from the processes of devitrification, leaching, weathering, erosion, and interactions with meteoric waters.
The SPB uranium district underwent a series of hydrothermal activities, which altered the rock and the uranium minerals. After the folding of the basal rocks layer, the volcanic pack was discordantly generated in the Peña Blanca uranium district between 44 and 37 M.a. [
27]. These volcanic rocks are contemporaneous to the Superior Volcanic Series. Subsequently, a regional extension event induced the formation of the Basin and Range Province (31–28 M.a.). At the same time, peralkaline rocks were emplaced from the Nido Block to Texas. Tectonism engendered a stepped slope from west to east, resulting in a hydraulic gradient that allowed, together with meteoric water, the movement of uranium through the clastic and volcanoclastic sedimentary horizons, from the Nido Block, crossing the Encinillas Basin, to the uranium district of Peña Blanca, where the uranium was reduced thanks to favorable conditions allowing it to precipitate and/or to concentrate.
A hydrothermal origin for the uranium in SPB, from deeper rocks present in situ, via various geological stages and events was also proposed [
19]. In the case of the Nopal I deposits, the combination of hydrothermal activity (evidenced by the presence of kaolin), supergene activity (evidenced by the devitrification of the vitrophyres and the presence of montmorillonite), and tectonic activity (evidenced by the filling of carbonates of the faults) may be responsible for the precipitation of uranium minerals.
The Margaritas Mine (
Figure 4) is located in the central part of the Peña Blanca uranium district at coordinates 396,120 east and 3,222,175 north. According to [
17], it covers three mineralized horizons: the lower part of the Escuadra Formation, the Chontes Formation and the top of the Nopal Formation. The Margaritas deposit is housed in gravel limited by tilted horsts dipping towards the east. In the 1980s, 1224 tons of U
3O
8 were estimated to reside in this deposit [
28].
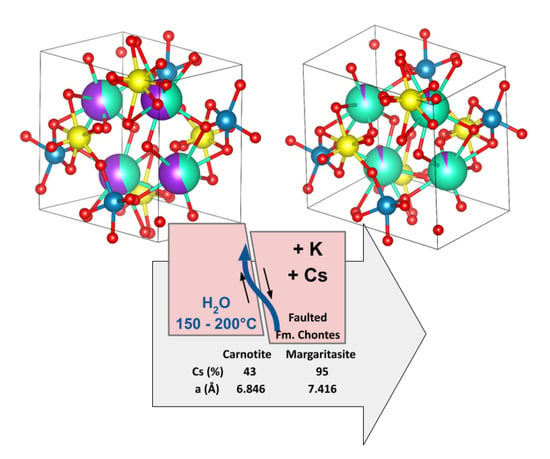
6. Summary
Margaritasite is a rare uranyl vanadate, found only in the Margaritas mine, in the Chontes Formation, at Sierra Peña Blanca, Chihuahua, Mexico.
Here, the presence of margaritasite–carnotite, issued from the SPB deposit, has been confirmed through a detailed crystallochemical characterization of representative samples, using updated, independent, and complementary techniques.
A synergetic use of high-resolution XRD, SEM, EDX, SAED, and XAS shows that this natural margaritasite–carnotite consists of a mixture of practically pure margaritasite and a solid solution of margaritasite–carnotite, with variable amounts of cesium. Detailed descriptions of both phases are provided in this work. The paragenesis concept enables a model that justifies the presence of cesium in natural margaritasite.