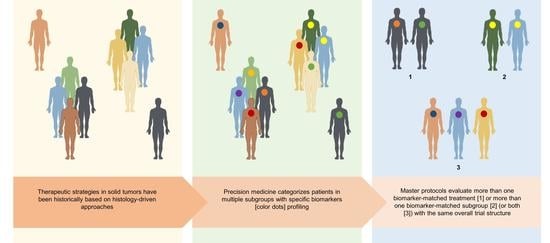
Master Protocols for Precision Medicine in Oncology: Overcoming Methodology of Randomized Clinical Trials
Abstract
:1. Introduction
2. Umbrella Trials
3. Basket Trials
4. Platform Trials
5. Opportunities and Limitations of Biomarker-Driven Master Protocols
6. Statistical Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Maio, M.; Gallo, C.; De Maio, E.; Morabito, A.; Piccirillo, M.C.; Gridelli, C.; Perrone, F. Methodological aspects of lung cancer clinical trials in the era of targeted agents. Lung Cancer 2010, 67, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Lu, T.-P.; Chen, D.-T.; Wang, S.-J. Biomarker adaptive designs in clinical trials. Transl. Cancer Res. 2014, 3, 279–292. [Google Scholar]
- Kumar-Sinha, C.; Chinnaiyan, A.M. Precision oncology in the age of integrative genomics. Nat. Biotechnol. 2018, 36, 46–60. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Grabowski, H.G. Economics of New Oncology Drug Development. J. Clin. Oncol. 2007, 25, 209–216. [Google Scholar] [CrossRef]
- Pascarella, G.; Capasso, A.; Nardone, A.; Triassi, M.; Pignata, S.; Arenare, L.; Ascierto, P.; Curvietto, M.; Maiolino, P.; D’Aniello, R.; et al. Costs of clinical trials with anticancer biological agents in an Oncologic Italian Cancer Center using the activity-based costing methodology. PLoS ONE 2019, 14, e0210330. [Google Scholar] [CrossRef]
- D’Ambrosio, F.; De Feo, G.; Botti, G.; Capasso, A.; Pignata, S.; Maiolino, P.; Triassi, M.; Nardone, A.; Perrone, F.; Piezzo, M.; et al. Clinical trials and drug cost savings for Italian health service. BMC Health Serv. Res. 2020, 20, 1089. [Google Scholar] [CrossRef]
- Masucci, L.; Torres, S.; Eisen, A.; Trudeau, M.; Tyono, I.; Saunders, H.; Chan, K.W.; Isaranuwatchai, W. Cost–Utility Analysis of 21-Gene Assay for Node-Positive Early Breast Cancer. Curr. Oncol. 2019, 26, 307–318. [Google Scholar] [CrossRef] [Green Version]
- De Falco, V.; Poliero, L.; Vitiello, P.P.; Ciardiello, D.; Vitale, P.; Zanaletti, N.; Giunta, E.F.; Terminiello, M.; Caputo, V.; Carlino, F.; et al. Feasibility of next-generation sequencing in clinical practice: Results of a pilot study in the Department of Precision Medicine at the University of Campania ‘Luigi Vanvitelli’. ESMO Open 2020, 5, e000675. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, J.; LaVange, L.M. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N. Engl. J. Med. 2017, 377, 62–70. [Google Scholar] [CrossRef]
- Park, J.J.H.; Siden, E.; Zoratti, M.J.; Dron, L.; Harari, O.; Singer, J.; Lester, R.T.; Thorlund, K.; Mills, E.J. Systematic review of basket trials, umbrella trials, and platform trials: A landscape analysis of master protocols. Trials 2019, 20, 572. [Google Scholar] [CrossRef] [Green Version]
- Hirakawa, A.; Asano, J.; Sato, H.; Teramukai, S. Master protocol trials in oncology: Review and new trial designs. Contemp. Clin. Trials Commun. 2018, 12, 1–8. [Google Scholar] [CrossRef]
- Garralda, E.; Dienstmann, R.; Piris-Giménez, A.; Braña, I.; Rodon, J.; Tabernero, J. New clinical trial designs in the era of precision medicine. Mol. Oncol. 2019, 13, 549–557. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.T.; Kim, K.; Lee, H.; Kozarewa, I.; Mortimer, P.G.S.; Odegaard, J.I.; Harrington, E.A.; Lee, J.; Lee, T.; et al. Tumor Genomic Profiling Guides Patients with Metastatic Gastric Cancer to Targeted Treatment: The VIKTORY Umbrella Trial. Cancer Discov. 2019, 9, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Sands, J.; Mandrekar, S.J.; Oxnard, G.R.; Kozono, D.E.; Hillman, S.L.; Dahlberg, S.E.; Sun, Z.; Chaft, J.E.; Govindan, R.; Gerber, D.E.; et al. ALCHEMIST: Adjuvant targeted therapy or immunotherapy for high-risk resected NSCLC. J. Clin. Oncol. 2020, 38, TPS9077. [Google Scholar] [CrossRef]
- Govindan, R.; Mandrekar, S.J.; Gerber, D.E.; Oxnard, G.R.; Dahlberg, S.E.; Chaft, J.; Malik, S.; Mooney, M.; Abrams, J.S.; Jänne, P.A.; et al. ALCHEMIST Trials: A Golden Opportunity to Transform Outcomes in Early-Stage Non-Small Cell Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5439–5444. [Google Scholar] [CrossRef] [Green Version]
- Redman, M.W.; Papadimitrakopoulou, V.A.; Minichiello, K.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Vokes, E.; Ramalingam, S.; Leighl, N.; Bradley, J.; et al. Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): A biomarker-driven master protocol. Lancet Oncol. 2020, 21, 1589–1601. [Google Scholar] [CrossRef]
- Hofmann, D.; Nitz, U.; Gluz, O.; Kates, R.E.; Schinkoethe, T.; Staib, P.; Harbeck, N. WSG ADAPT—Adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: Study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials 2013, 14, 261. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.C.; Kingston, B.; Kilburn, L.S.; Kernaghan, S.; Wardley, A.M.; Macpherson, I.R.; Baird, R.D.; Roylance, R.; Stephens, P.; Oikonomidou, O.; et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): A multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020, 21, 1296–1308. [Google Scholar] [CrossRef]
- Renfro, L.A.; Sargent, D.J. Statistical controversies in clinical research: Basket trials, umbrella trials, and other master protocols: A review and examples. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 34–43. [Google Scholar] [CrossRef]
- Redig, A.J.; Jänne, P.A. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 975–977. [Google Scholar] [CrossRef] [Green Version]
- Mandrekar, S.J.; Dahlberg, S.E.; Simon, R. Improving Clinical Trial Efficiency: Thinking outside the Box. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e141–e147. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Delord, J.-P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.-A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Berry, S.M.; Connor, J.T.; Lewis, R.J. The platform trial: An efficient strategy for evaluating multiple treatments. JAMA 2015, 313, 1619–1620. [Google Scholar] [CrossRef]
- Angus, D.C.; Alexander, B.M.; Berry, S.; Buxton, M.; Lewis, R.; Paoloni, M.; Webb, S.A.R.; Arnold, S.; Barker, A.; Berry, D.A.; et al. Adaptive platform trials: Definition, design, conduct and reporting considerations. Nat. Rev. Drug Discov. 2019, 18, 797–807. [Google Scholar] [CrossRef]
- Beckman, R.A.; Antonijevic, Z.; Kalamegham, R.; Chen, C. Adaptive Design for a Confirmatory Basket Trial in Multiple Tumor Types Based on a Putative Predictive Biomarker. Clin. Pharmacol. Ther. 2016, 100, 617–625. [Google Scholar] [CrossRef]
- Millen, G.C.; Yap, C. Adaptive trial designs: What are multiarm, multistage trials? Arch. Dis. Child.-Educ. Pract. 2020, 105, 376–378. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Anderson, J.; Popert, R.J.; Sanders, K.; Morgan, R.C.; Stansfeld, J.; et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer—A multi-arm multi-stage randomised controlled trial. Clin. Oncol. 2008, 20, 577–581. [Google Scholar] [CrossRef]
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- Attard, G.; Sydes, M.R.; Mason, M.D.; Clarke, N.W.; Aebersold, D.; de Bono, J.S.; Dearnaley, D.P.; Parker, C.C.; Ritchie, A.W.S.; Russell, J.M.; et al. Combining Enzalutamide with Abiraterone, Prednisone, and Androgen Deprivation Therapy in the STAMPEDE Trial. Eur. Urol. 2014, 66, 799–802. [Google Scholar] [CrossRef] [Green Version]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomised controlled phase 3 trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [Green Version]
- STAMPEDE. Available online: http://www.stampedetrial.org/ (accessed on 15 September 2021).
- Adams, R.; Brown, E.; Brown, L.; Butler, R.; Falk, S.; Fisher, D.; Kaplan, R.; Quirke, P.; Richman, S.; Samuel, L.; et al. Inhibition of EGFR, HER2, and HER3 signalling in patients with colorectal cancer wild-type for BRAF, PIK3CA, KRAS, and NRAS (FOCUS4-D): A phase 2–3 randomised trial. Lancet Gastroenterol. Hepatol. 2018, 3, 162–171. [Google Scholar] [CrossRef] [Green Version]
- FOCUS4. Available online: http://www.focus4trial.org/ (accessed on 15 September 2021).
- Wang, H.; Yee, D. I-SPY 2: A Neoadjuvant Adaptive Clinical Trial Designed to Improve Outcomes in High-Risk Breast Cancer. Curr. Breast Cancer Rep. 2019, 11, 303–310. [Google Scholar] [CrossRef]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Carey, L.A.; Winer, E.P. I-SPY 2—Toward More Rapid Progress in Breast Cancer Treatment. N. Engl. J. Med. 2016, 375, 83–84. [Google Scholar] [CrossRef]
- Pusztai, L.; Yau, C.; Wolf, D.M.; Han, H.S.; Du, L.; Wallace, A.M.; String-Reasor, E.; Boughey, J.C.; Chien, A.J.; Elias, A.D.; et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell 2021, 39, 989–998. [Google Scholar] [CrossRef]
- Siden, E.G.; Park, J.J.H.; Zoratti, M.J.; Dron, L.; Harari, O.; Thorlund, K.; Mills, E.J. Reporting of master protocols towards a standardized approach: A systematic review. Contemp. Clin. Trials Commun. 2019, 15, 100406. [Google Scholar] [CrossRef]
- Teng, Z.; Tian, Y.; Liu, Y.; Liu, G. Seamless phase 2/3 oncology trial design with flexible sample size determination. Stat. Med. 2020, 39, 2373–2386. [Google Scholar] [CrossRef]
- Teng, Z.; Liang, L.; Liu, G.; Liu, Y. Optimal seamless phase 2/3 oncology trial designs based on Probability of Success (PoS). Stat. Med. 2018, 37, 4097–4113. [Google Scholar] [CrossRef]
- Hobbs, B.P.; Barata, P.C.; Kanjanapan, Y.; Paller, C.J.; Perlmutter, J.; Pond, G.R.; Prowell, T.M.; Rubin, E.H.; Seymour, L.K.; Wages, N.A.; et al. Seamless Designs: Current Practice and Considerations for Early-Phase Drug Development in Oncology. JNCI J. Natl. Cancer Inst. 2019, 111, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Janiaud, P.; Serghiou, S.; Ioannidis, J.P.A. New clinical trial designs in the era of precision medicine: An overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treat. Rev. 2019, 73, 20–30. [Google Scholar] [CrossRef]
- Di Liello, R.; Cimmino, F.; Simón, S.; Giunta, E.F.; De Falco, V.; Martín-Martorell, P. Role of liquid biopsy for thoracic cancers immunotherapy. Explor. Target. Anti-Tumor Ther. 2020, 1, 183–199. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef]
- Hunter, D.J.; D’Agostino, R.B. Let’s Not Put All Our Eggs in One Basket. N. Engl. J. Med. 2015, 373, 691–693. [Google Scholar] [CrossRef]
- Al-Mekhlafi, A.; Becker, T.; Klawonn, F. Sample size and performance estimation for biomarker combinations based on pilot studies with small sample sizes. Commun. Stat.-Theory Methods 2020, 1–15. [Google Scholar] [CrossRef]
- Hua, S.Y.; Xu, S.; D’Agostino Sr., R.B. Multiplicity adjustments in testing for bioequivalence. Stat. Med. 2015, 34, 215–231. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Yuan, S.; Antonijevic, Z.; Kalamegham, R.; Beckman, R.A. Statistical Design and Considerations of a Phase 3 Basket Trial for Simultaneous Investigation of Multiple Tumor Types in One Study. Stat. Biopharm. Res. 2016, 8, 248–257. [Google Scholar] [CrossRef]
- Gyawali, B.; Hey, S.P.; Kesselheim, A.S. Evaluating the evidence behind the surrogate measures included in the FDA’s table of surrogate endpoints as supporting approval of cancer drugs. EClinicalMedicine 2020, 21, 100332. [Google Scholar] [CrossRef]
- Paoletti, X.; Lewsley, L.-A.; Daniele, G.; Cook, A.; Yanaihara, N.; Tinker, A.; Kristensen, G.; Ottevanger, P.B.; Aravantinos, G.; Miller, A.; et al. Assessment of Progression-Free Survival as a Surrogate End Point of Overall Survival in First-Line Treatment of Ovarian Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e1918939. [Google Scholar] [CrossRef]
- Goldman, B.; LeBlanc, M.; Crowley, J. Interim futility analysis with intermediate endpoints. Clin. Trials 2008, 5, 14–22. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Liello, R.; Piccirillo, M.C.; Arenare, L.; Gargiulo, P.; Schettino, C.; Gravina, A.; Perrone, F. Master Protocols for Precision Medicine in Oncology: Overcoming Methodology of Randomized Clinical Trials. Life 2021, 11, 1253. https://0-doi-org.brum.beds.ac.uk/10.3390/life11111253
Di Liello R, Piccirillo MC, Arenare L, Gargiulo P, Schettino C, Gravina A, Perrone F. Master Protocols for Precision Medicine in Oncology: Overcoming Methodology of Randomized Clinical Trials. Life. 2021; 11(11):1253. https://0-doi-org.brum.beds.ac.uk/10.3390/life11111253
Chicago/Turabian StyleDi Liello, Raimondo, Maria Carmela Piccirillo, Laura Arenare, Piera Gargiulo, Clorinda Schettino, Adriano Gravina, and Francesco Perrone. 2021. "Master Protocols for Precision Medicine in Oncology: Overcoming Methodology of Randomized Clinical Trials" Life 11, no. 11: 1253. https://0-doi-org.brum.beds.ac.uk/10.3390/life11111253