Fall Treatment with Fumagillin Contributes to an Overwinter Shift in Vairimorpha Species Prevalence in Honey Bee Colonies in Western Canada
Abstract
:1. Introduction
2. Materials and Methods
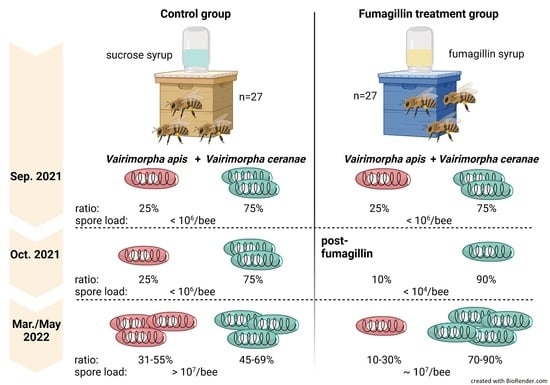
2.1. Experimental Design
2.2. Vairimorpha Colony Spore Load
2.3. Molecular Determination of Vairimorpha Species Prevalence
2.4. Colony Weight and Cluster Size
2.5. Varroa Infestation
2.6. Temperature Monitoring
2.7. Statistical Analysis
3. Results
3.1. Fumagillin Consumption
3.2. Colony Vairimorpha Infection
3.3. Vairimorpha spp. Prevalence
3.4. Colony Strength (Cluster Size) and Weight
3.5. Varroa Infestation
3.6. Overwinter Survival and Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Association of Professional Apiculturists Statement on Honey Bee Wintering Losses in Canada. 2022. Available online: https://capabees.com/shared/CAPA-Statement-on-Colony-Losses-2021-2022-FV.pdf (accessed on 3 February 2023).
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; del Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How Natural Infection by Nosema ceranae Causes Honeybee Colony Collapse: Natural Nosema ceranae Infection. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef]
- Ellis, J.D.; Evans, J.D.; Pettis, J. Colony Losses, Managed Colony Population Decline, and Colony Collapse Disorder in the United States. J. Apic. Res. 2010, 49, 134–136. [Google Scholar] [CrossRef]
- VanEngelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A Survey of Honey Bee Colony Losses in the US, Fall 2007 to Spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.-P.; vanEngelsdorp, D. Drivers of Colony Losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef]
- Fries, I. Nosema apis—A Parasite in the Honey Bee Colony. Bee World 1993, 74, 5–19. [Google Scholar] [CrossRef]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. pp. (Microspora, Nosematidae), Morphological and Molecular Characterization of a Microsporidian Parasite of the Asian Honey Bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Huang, W.-F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A Formal Redefinition of the Genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and Reassignment of Species Based on Molecular Phylogenetics. J. Invertebr. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef] [PubMed]
- Kudo, R. Notes on Nosema apis Zander. J. Parasitol. 1920, 7, 85–90. [Google Scholar] [CrossRef]
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae, a New Microsporidian Parasite in Honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. Infection and Its Negative Effects on Honey Bees (Apis mellifera iberiensis) at the Colony Level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef]
- Klee, J.; Besana, A.M.; Genersch, E.; Gisder, S.; Nanetti, A.; Tam, D.Q.; Chinh, T.X.; Puerta, F.; Ruz, J.M.; Kryger, P.; et al. Widespread Dispersal of the Microsporidian Nosema ceranae, an Emergent Pathogen of the Western Honey Bee, Apis mellifera. J. Invertebr. Pathol. 2007, 96, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae Is a Long-Present and Wide-Spread Microsporidian Infection of the European Honey Bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef]
- Williams, G.R.; Shafer, A.B.A.; Rogers, R.E.L.; Shutler, D.; Stewart, D.T. First Detection of Nosema ceranae, a Microsporidian Parasite of European Honey Bees (Apis mellifera), in Canada and Central USA. J. Invertebr. Pathol. 2008, 97, 189–192. [Google Scholar] [CrossRef]
- Traver, B.E.; Fell, R.D. A Scientific Note: Survey for Nosema spp. in Preserved Apis spp. Apidologie 2015, 46, 194–196. [Google Scholar] [CrossRef]
- Currie, R.W.; Pernal, S.F.; Guzmán-Novoa, E. Honey Bee Colony Losses in Canada. J. Apic. Res. 2010, 49, 104–106. [Google Scholar] [CrossRef]
- Emsen, B.; Guzman-Novoa, E.; Hamiduzzaman, M.M.; Eccles, L.; Lacey, B.; Ruiz-Pérez, R.A.; Nasr, M. Higher Prevalence and Levels of Nosema ceranae than Nosema apis Infections in Canadian Honey Bee Colonies. Parasitol. Res. 2016, 115, 175–181. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Bailón, E.G.; Martínez-Salvador, A.; Prieto, L.; Meana, A.; Higes, M. Microsporidia Infecting Apis mellifera: Coexistence or Competition. Is Nosema ceranae Replacing Nosema apis? Environ. Microbiol. 2012, 14, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Forsgren, E.; Fries, I. Comparative Virulence of Nosema ceranae and Nosema apis in Individual European Honey Bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef]
- Huang, W.-F.; Solter, L.; Aronstein, K.; Huang, Z. Infectivity and Virulence of Nosema ceranae and Nosema apis in Commercially Available North American Honey Bees. J. Invertebr. Pathol. 2015, 124, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Milbrath, M.O.; van Tran, T.; Huang, W.-F.; Solter, L.F.; Tarpy, D.R.; Lawrence, F.; Huang, Z.Y. Comparative Virulence and Competition between Nosema apis and Nosema ceranae in Honey Bees (Apis mellifera). J. Invertebr. Pathol. 2015, 125, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sinpoo, C.; Paxton, R.J.; Disayathanoowat, T.; Krongdang, S.; Chantawannakul, P. Impact of Nosema ceranae and Nosema apis on Individual Worker Bees of the Two Host Species (Apis cerana and Apis mellifera) and Regulation of Host Immune Response. J. Insect Physiol. 2018, 105, 1–8. [Google Scholar] [CrossRef]
- Dussaubat, C.; Sagastume, S.; Gómez-Moracho, T.; Botías, C.; García-Palencia, P.; Martín-Hernández, R.; Le Conte, Y.; Higes, M. Comparative Study of Nosema ceranae (Microsporidia) Isolates from Two Different Geographic Origins. Vet. Microbiol. 2013, 162, 670–678. [Google Scholar] [CrossRef]
- Antúnez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune Suppression in the Honey Bee (Apis mellifera) Following Infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Natsopoulou, M.E.; McMahon, D.P.; Doublet, V.; Bryden, J.; Paxton, R.J. Interspecific Competition in Honeybee Intracellular Gut Parasites Is Asymmetric and Favours the Spread of an Emerging Infectious Disease. Proc. R. Soc. B Biol. Sci. 2015, 282, 20141896. [Google Scholar] [CrossRef]
- Pettis, J.S.; vanEngelsdorp, D.; Johnson, J.; Dively, G. Pesticide Exposure in Honey Bees Results in Increased Levels of the Gut Pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Toplak, I.; Jamnikar Ciglenečki, U.; Aronstein, K.; Gregorc, A. Chronic Bee Paralysis Virus and Nosema ceranae Experimental Co-Infection of Winter Honey Bee Workers (Apis mellifera L.). Viruses 2013, 5, 2282–2297. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor Is the Main Culprit for the Death and Reduced Populations of Overwintered Honey Bee (Apis mellifera) Colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Holt, H.L.; Grozinger, C.M. Approaches and Challenges to Managing Nosema (Microspora: Nosematidae) Parasites in Honey Bee (Hymenoptera: Apidae) Colonies. J. Econ. Entomol. 2016, 109, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae Escapes Fumagillin Control in Honey Bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef]
- Alberta Agriculture and Forestry Honey Bee Pests and Diseases—Best Management Practices. 2020. Available online: https://open.alberta.ca/publications/9781460147696 (accessed on 31 January 2024).
- Bourgeois, A.L.; Rinderer, T.E.; Beaman, L.D.; Danka, R.G. Genetic Detection and Quantification of Nosema apis and N. ceranae in the Honey Bee. J. Invertebr. Pathol. 2010, 103, 53–58. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard Methods for Nosema Research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Biganski, S.; Lester, T.; Obshta, O.; Jose, M.S.; Thebeau, J.M.; Masood, F.; Silva, M.C.B.; Camilli, M.P.; Raza, M.F.; Zabrodski, M.W.; et al. Comparison of Individual and Pooled Sampling Methods for Estimation of Vairimorpha (Nosema) spp. Levels in Experimentally Infected Honey Bee Colonies. J. Vet. Diagn. Investig. 2023, 35, 639–644. [Google Scholar] [CrossRef]
- Echazarreta, J.M.; Delhey, V.K.; Pellegrini, C.N.; Gallez, L.M. Variability of Vairimorpha (=Nosema) ceranae Infection Level in Individual Honey Bees and Its Implications on the Pooled Sample Size. J. Apic. Res. 2023, 1–8. [Google Scholar] [CrossRef]
- Katznelson, H.; Jamieson, C.A. Control of Nosema Disease of Honeybees with Fumagillin. Science 1952, 115, 70–71. [Google Scholar] [CrossRef]
- Webster, T.C. Fumagillin Affects Nosema apis and Honey Bees (Hymonopterai Apidae). J. Econ. Entomol. 1994, 87, 601–604. [Google Scholar] [CrossRef]
- McCallum, R.; Olmstead, S.; Shaw, J.; Glasgow, K. Evaluating Efficacy of Fumagilin-B® Against Nosemosis and Tracking Seasonal Trends of Nosema spp. in Nova Scotia Honey Bee Colonies. J. Apic. Sci. 2020, 64, 277–286. [Google Scholar] [CrossRef]
- Punko, R.N.; Currie, R.W.; Nasr, M.E.; Hoover, S.E. Effect of Fumagilin-B Treatment Timing on Nosema (Vairimorpha spp.; Microspora: Nosematidae) Abundance and Honey Bee (Hymenoptera: Apidae) Colonies under Winter Management in the Canadian Prairies. J. Econ. Entomol. 2023, 116, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Martín-Hernández, R.; Bernal, J.L.; Bernal, J. The Stability and Effectiveness of Fumagillin in Controlling Nosema ceranae (Microsporidia) Infection in Honey Bees (Apis mellifera) under Laboratory and Field Conditions. Apidologie 2011, 42, 364–377. [Google Scholar] [CrossRef]
- Williams, G.R.; Shutler, D.; Little, C.M.; Burgher-Maclellan, K.L.; Rogers, R.E.L. The Microsporidian Nosema ceranae, the Antibiotic Fumagilin-B®, and Western Honey Bee (Apis mellifera) Colony Strength. Apidologie 2011, 42, 15–22. [Google Scholar] [CrossRef]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E.L. Does Fumagillin Control the Recently Detected Invasive Parasite Nosema ceranae in Western Honey Bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Genersch, E. Identification of Candidate Agents Active against N. ceranae Infection in Honey Bees: Establishment of a Medium Throughput Screening Assay Based on N. ceranae Infected Cultured Cells. PLoS ONE 2015, 10, e0117200. [Google Scholar] [CrossRef]
- van den Heever, J.P.; Thompson, T.S.; Otto, S.J.G.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. The Effect of Dicyclohexylamine and Fumagillin on Nosema ceranae-Infected Honey Bee (Apis mellifera) Mortality in Cage Trial Assays. Apidologie 2016, 47, 663–670. [Google Scholar] [CrossRef]
- Peirson, M.; Pernal, S.F. A Systematic Review of Fumagillin Field Trials for the Treatment of Nosema Disease in Honeybee Colonies. Insects 2024, 15, 29. [Google Scholar] [CrossRef]
- Gary, N.E.; Lorenzen, K. Vacuum Devices for Capturing and Partitioning Commingled Subpopulations of Honey Bees (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 1990, 83, 1152–1154. [Google Scholar] [CrossRef]
- Traver, B.E.; Fell, R.D. Prevalence and Infection Intensity of Nosema in Honey Bee (Apis mellifera L.) Colonies in Virginia. J. Invertebr. Pathol. 2011, 107, 43–49. [Google Scholar] [CrossRef]
- Roetschi, A.; Berthoud, H.; Kuhn, R.; Imdorf, A. Infection Rate Based on Quantitative Real-Time PCR of Melissococcus plutonius, the Causal Agent of European Foulbrood, in Honeybee Colonies before and after Apiary Sanitation. Apidologie 2008, 39, 362–371. [Google Scholar] [CrossRef]
- Nasr, M.E.; Thorp, R.W.; Tyler, T.L.; Briggs, D.L. Estimating Honey Bee (Hymenoptera: Apidae) Colony Strength by a Simple Method: Measuring Cluster Size. J. Econ. Entomol. 1990, 83, 748–754. [Google Scholar] [CrossRef]
- Wood, S.C.; Kozii, I.V.; Koziy, R.V.; Epp, T.; Simko, E. Comparative Chronic Toxicity of Three Neonicotinoids on New Zealand Packaged Honey Bees. PLoS ONE 2018, 13, e0190517. [Google Scholar] [CrossRef]
- De Jong, D.; De Andrea Roma, D.; Goncalves, L.S. A Comparative Analysis of Shaking Solutions for the Detection of Varroa jacobsoni on Adult Honeybees. Apidologie 1982, 13, 297–306. [Google Scholar] [CrossRef]
- Lee, K.V.; Moon, R.D.; Burkness, E.C.; Hutchison, W.D.; Spivak, M. Practical Sampling Plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) Colonies and Apiaries. J. Econ. Entomol. 2010, 103, 1039–1050. [Google Scholar] [CrossRef]
- Meikle, W.G.; Weiss, M.; Stilwell, A.R. Monitoring Colony Phenology Using Within-Day Variability in Continuous Weight and Temperature of Honey Bee Hives. Apidologie 2016, 47, 1–14. [Google Scholar] [CrossRef]
- Prouty, C.; Jack, C.; Sagili, R.; Ellis, J.D. Evaluating the Efficacy of Common Treatments Used for Vairimorpha (Nosema) spp. Control. Appl. Sci. 2023, 13, 1303. [Google Scholar] [CrossRef]
- Li, J.H.; Evans, J.D.; Li, W.F.; Zhao, Y.Z.; DeGrandi-Hoffman, G.; Huang, S.K.; Li, Z.G.; Hamilton, M.; Chen, Y.P. New Evidence Showing That the Destruction of Gut Bacteria by Antibiotic Treatment Could Increase the Honey Bee’s Vulnerability to Nosema Infection. PLoS ONE 2017, 12, e0187505. [Google Scholar] [CrossRef]
- Nozal, M.J.; Bernal, J.L.; Martín, M.T.; Bernal, J.; Álvaro, A.; Martín, R.; Higes, M. Trace Analysis of Fumagillin in Honey by Liquid Chromatography-Diode Array–Electrospray Ionization Mass Spectrometry. J. Chromatogr. A 2008, 1190, 224–231. [Google Scholar] [CrossRef]
- Emsen, B.; De la Mora, A.; Lacey, B.; Eccles, L.; Kelly, P.G.; Medina-Flores, C.A.; Petukhova, T.; Morfin, N.; Guzman-Novoa, E. Seasonality of Nosema ceranae Infections and Their Relationship with Honey Bee Populations, Food Stores, and Survivorship in a North American Region. Vet. Sci. 2020, 7, 131. [Google Scholar] [CrossRef]
- Copley, T.R.; Chen, H.; Giovenazzo, P.; Houle, E.; Jabaji, S.H. Prevalence and Seasonality of Nosema Species in Québec Honey Bees. Can. Entomol. 2012, 144, 577–588. [Google Scholar] [CrossRef]
- Mendoza, Y.; Diaz-Cetti, S.; Ramallo, G.; Santos, E.; Porrini, M.; Invernizzi, C. Nosema ceranae Winter Control: Study of the Effectiveness of Different Fumagillin Treatments and Consequences on the Strength of Honey Bee (Hymenoptera: Apidae) Colonies. J. Econ. Entomol. 2016, 110, 1–5. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biganski, S.; Obshta, O.; Kozii, I.; Koziy, R.; Zabrodski, M.W.; Jose, M.S.; Thebeau, J.M.; Silva, M.C.B.; Raza, M.F.; Masood, F.; et al. Fall Treatment with Fumagillin Contributes to an Overwinter Shift in Vairimorpha Species Prevalence in Honey Bee Colonies in Western Canada. Life 2024, 14, 373. https://0-doi-org.brum.beds.ac.uk/10.3390/life14030373
Biganski S, Obshta O, Kozii I, Koziy R, Zabrodski MW, Jose MS, Thebeau JM, Silva MCB, Raza MF, Masood F, et al. Fall Treatment with Fumagillin Contributes to an Overwinter Shift in Vairimorpha Species Prevalence in Honey Bee Colonies in Western Canada. Life. 2024; 14(3):373. https://0-doi-org.brum.beds.ac.uk/10.3390/life14030373
Chicago/Turabian StyleBiganski, Sarah, Oleksii Obshta, Ivanna Kozii, Roman Koziy, Michael W. Zabrodski, Midhun S. Jose, Jenna M. Thebeau, Marina C. B. Silva, Muhammad F. Raza, Fatima Masood, and et al. 2024. "Fall Treatment with Fumagillin Contributes to an Overwinter Shift in Vairimorpha Species Prevalence in Honey Bee Colonies in Western Canada" Life 14, no. 3: 373. https://0-doi-org.brum.beds.ac.uk/10.3390/life14030373