The Interrelationship between Ventilatory Inefficiency and Left Ventricular Ejection Fraction in Terms of Cardiovascular Outcomes in Heart Failure Outpatients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. CPET Procedures
2.3. Outcome Analysis
2.4. Statistical Analyses
3. Results
3.1. Baseline Clinical and Pharmacological Characteristics by LVEF
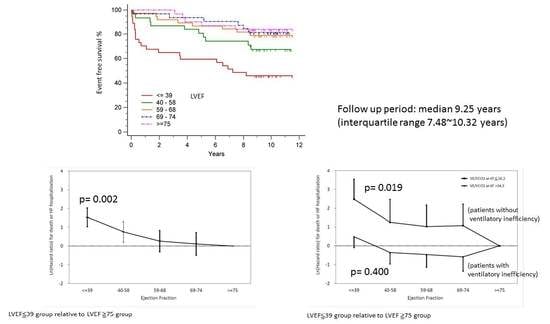
3.2. Outcomes by LVEF
3.3. Univariate and Multivariate Analysis of Predictors of Major Cardiovascular Events
3.4. Adjust Hazard Ratio Associated with LVEF for Major Cardiovascular Events by Baseline LVEF Category Relative to LVEF ≥ 75
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.M.; Fang, Y.N.; Wang, L.Y.; Wu, M.K.; Wu, P.J.; Yang, T.H.; Chen, Y.L.; Hang, C.L. Impact of multi-disciplinary treatment strategy on systolic heart failure outcome. BMC Cardiovasc. Disord. 2019, 19, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, 1810–1852. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.M.; Feinglass, J.; Lee, P.I.; Mehta, S.; Schmitt, B.; Lefevre, F.; Gheorghiade, M. Systolic function, readmission rates, and survival among consecutively hospitalized patients with congestive heart failure. Am. Heart J. 1997, 134, 728–736. [Google Scholar] [CrossRef]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef] [Green Version]
- Pocock, S.J.; Wang, D.; Pfeffer, M.A.; Yusuf, S.; McMurray, J.J.; Swedberg, K.B.; Ostergren, J.; Michelson, E.L.; Pieper, K.S.; Granger, C.B. Predictors of mortality and morbidity in patients with chronic heart failure. Eur. Heart J. 2006, 27, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Paolillo, S.; Agostoni, P. Prognostic Role of Cardiopulmonary Exercise Testing in Clinical Practice. Ann. Am. Thorac. Soc. 2017, 14, S53–S58. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart. Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Poggio, R.; Arazi, H.C.; Giorgi, M.; Miriuka, S.G. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: A meta-analysis of the published literature. Am. Heart J. 2010, 160, 1004–1014. [Google Scholar] [CrossRef]
- Kleber, F.X.; Vietzke, G.; Wernecke, K.D.; Bauer, U.; Opitz, C.; Wensel, R.; Sperfeld, A.; Glaser, S. Impairment of ventilatory efficiency in heart failure: Prognostic impact. Circulation 2000, 101, 2803–2809. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K.; Zhang, Y.Y.; Gitt, A.; Belardinelli, R.; Koike, A.; Lubarsky, L.; Agostoni, P.G. Lung function and exercise gas exchange in chronic heart failure. Circulation 1997, 96, 2221–2227. [Google Scholar] [CrossRef]
- Sun, X.G.; Hansen, J.E.; Garatachea, N.; Storer, T.W.; Wasserman, K. Ventilatory efficiency during exercise in healthy subjects. Am. J. Respir. Crit. Care Med. 2002, 166, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Arena, R.; Cahalin, L.P.; Labate, V.; Guazzi, M. Cardiopulmonary Exercise Testing in Heart Failure. Curr. Probl. Cardiol. 2015, 40, 322–372. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.P.; Snyder, E.M.; Johnson, B.D. Exercise-disordered breathing in chronic heart failure. Exerc. Sport Sci. Rev. 2006, 34, 194–201. [Google Scholar] [CrossRef]
- Goodlin, S.J. Palliative care in congestive heart failure. J. Am. Coll. Cardiol. 2009, 54, 386–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, A.; Arbex, F.F.; Sperandio, P.A.; Souza, A.; Biazzim, L.; Mancuso, F.; Berton, D.C.; Hochhegger, B.; Alencar, M.C.N.; Nery, L.E.; et al. Excess Ventilation in Chronic Obstructive Pulmonary Disease-Heart Failure Overlap. Implications for Dyspnea and Exercise Intolerance. Am. J. Respir. Crit. Care Med. 2017, 196, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.L., Jr. Gas exchange efficiency in congestive heart failure II. Circulation 2001, 103, 916–918. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.L., Jr. Gas exchange efficiency in congestive heart failure. Circulation 2000, 101, 2774–2776. [Google Scholar] [CrossRef] [Green Version]
- Tsujinaga, S.; Iwano, H.; Chiba, Y.; Ishizaka, S.; Sarashina, M.; Murayama, M.; Nakabachi, M.; Nishino, H.; Yokoyama, S.; Okada, K.; et al. Heart Failure With Preserved Ejection Fraction vs. Reduced Ejection Fraction―Mechanisms of Ventilatory Inefficiency During Exercise in Heart Failure―. Circ. Rep. 2020. [Google Scholar] [CrossRef] [Green Version]
- Van Iterson, E.H.; Johnson, B.D.; Borlaug, B.A.; Olson, T.P. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur. J. Heart Fail. 2017, 19, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guazzi, M.; Marenzi, G.; Alimento, M.; Contini, M.; Agostoni, P. Improvement of alveolar-capillary membrane diffusing capacity with enalapril in chronic heart failure and counteracting effect of aspirin. Circulation 1997, 95, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Apostolo, A.; Cattadori, G.; Salvioni, E.; Berna, G.; Antonioli, L.; Vignati, C.; Schina, M.; Sciomer, S.; Bussotti, M.; et al. Effects of beta-blockers on ventilation efficiency in heart failure. Am. Heart J. 2010, 159, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Contini, M.; Apostolo, A.; Cattadori, G.; Paolillo, S.; Iorio, A.; Bertella, E.; Salvioni, E.; Alimento, M.; Farina, S.; Palermo, P.; et al. Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure: The CARNEBI trial. Int. J. Cardiol. 2013, 168, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Reese, V.; Shabetai, R.; Wagner, P.D.; Richardson, R.S. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: The role of skeletal muscle convective and diffusive oxygen transport. J. Am. Coll. Cardiol. 2011, 58, 1353–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parati, G.; Malfatto, G.; Boarin, S.; Branzi, G.; Caldara, G.; Giglio, A.; Bilo, G.; Ongaro, G.; Alter, A.; Gavish, B.; et al. Device-guided paced breathing in the home setting: Effects on exercise capacity, pulmonary and ventricular function in patients with chronic heart failure: A pilot study. Circ. Heart Fail. 2008, 1, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, L.; Sleight, P.; Bandinelli, G.; Cencetti, S.; Fattorini, L.; Wdowczyc-Szulc, J.; Lagi, A. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: Comparative study. BMJ 2001, 323, 1446–1449. [Google Scholar] [CrossRef] [Green Version]
- Chua, T.P.; Ponikowski, P.P.; Harrington, D.; Chambers, J.; Coats, A.J. Contribution of peripheral chemoreceptors to ventilation and the effects of their suppression on exercise tolerance in chronic heart failure. Heart 1996, 76, 483–489. [Google Scholar] [CrossRef]
- Wensel, R.; Georgiadou, P.; Francis, D.P.; Bayne, S.; Scott, A.C.; Genth-Zotz, S.; Anker, S.D.; Coats, A.J.; Piepoli, M.F. Differential contribution of dead space ventilation and low arterial pCO2 to exercise hyperpnea in patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2004, 93, 318–323. [Google Scholar] [CrossRef]
- Mehra, M.R.; Kobashigawa, J.; Starling, R.; Russell, S.; Uber, P.A.; Parameshwar, J.; Mohacsi, P.; Augustine, S.; Aaronson, K.; Barr, M. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates--2006. J. Heart. Lung Transplant. 2006, 25, 1024–1042. [Google Scholar] [CrossRef]
- Lewis, E.F.; Moye, L.A.; Rouleau, J.L.; Sacks, F.M.; Arnold, J.M.; Warnica, J.W.; Flaker, G.C.; Braunwald, E.; Pfeffer, M.A.; Study, C. Predictors of late development of heart failure in stable survivors of myocardial infarction: The CARE study. J. Am. Coll. Cardiol. 2003, 42, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Lupón, J.; Díez-López, C.; de Antonio, M.; Domingo, M.; Zamora, E.; Moliner, P.; González, B.; Santesmases, J.; Troya, M.I.; Bayés-Genís, A. Recovered heart failure with reduced ejection fraction and outcomes: A prospective study. Eur. J. Heart Fail. 2017, 19, 1615–1623. [Google Scholar] [CrossRef] [PubMed]




| Variables | All Patients (n = 169) | LVEF ≤39% (37) | LVEF 40–58% (31) | LVEF 59–68% (38) | LVEF 69–74% (32) | LVEF ≥75% (31) | p Value |
|---|---|---|---|---|---|---|---|
| Age | 55.7 ± 13.5 | 50.9 ± 14.7 | 59.6 ± 12.3 | 54.3 ± 12.8 | 57.1 ± 14.6 | 57.7 ± 11.7 | 0.097 |
| Male | 121 (71.6%) | 34 (91.9%) | 23 (74.2%) | 27 (71.1%) | 17 (53.1%) | 20 (64.5%) | 0.008 |
| Lung disease Both (%) | 79 (46.7%) | 20 (54.1%) | 17 (54.8%) | 15 (39.5%) | 15 (46.9%) | 12 (38.7%) | 0.522 |
| Obstructive lung (%) | 13 (7.7%) | 4 (10.8%) | 4 (12.9%) | 1 (2.6%) | 3 (9.4%) | 1 (3.2%) | 0.396 |
| Restrictive lung (%) | 66 (39.1%) | 16 (43.2%) | 13 (41.9%) | 14 (36.8%) | 12 (37.5%) | 11 (35.5%) | 0.956 |
| Hypertension (%) | 99 (58.6%) | 13 (35.1%) | 23 (74.2%) | 23 (60.5%) | 19 (65.5%) | 21 (72.4%) | 0.006 |
| Diabetes (%) | 37 (22.7%) | 9 (24.3%) | 10 (32.3%) | 10 (26.3%) | 4 (13.8%) | 4 (14.3%) | 0.355 |
| Smoking (%) | 39 (23.5%) | 16 (43.2%) | 8 (25.8%) | 7 (18.4) | 5 (16.1%) | 3 (10.3%) | 0.015 |
| Ischemic stroke (%) | 9 (5.6%) | 0 (0%) | 1(3.2%) | 2 (5.3%) | 2 (6.9%) | 4 (14.3%) | 0.158 |
| Ischemic CM (%) | 33 (19.5%) | 15 (40.5%) | 10 (32.3%) | 2 (5.3%) | 4 (12.5%) | 2 (6.5%) | <0.0001 |
| Valvular CM (%) | 22 (13.0%) | 3 (8.1%) | 6 (19.4%) | 4 (10.5%) | 3 (9.4%) | 6 (19.4%) | 0.497 |
| Dilated CM (%) | 24 (14.2%) | 15 (40.5%) | 7 (22.6%) | 2 (5.3%) | 0 (0%) | 0 (0%) | <0.0001 |
| Prior PCI (%) | 29 (17.2%) | 13 (35.1%) | 9 (29.0%) | 2 (5.3%) | 4 (12.5%) | 1 (3.2%) | 0.001 |
| Medication | |||||||
| Beta-blocker (%) | 97 (58.4%) | 30 (81.1%) | 25 (80.6%) | 19 (50.0%) | 12 (38.7%) | 11 (37.9%) | <0.0001 |
| ACEI/ARB (%) | 114 (67.5%) | 32 (86.5%) | 28 (90.3%) | 21 (55.3%) | 15 (46.9%) | 18 (58.1%) | <0.0001 |
| DHP Ca+ channel blocker (%) | 36 (21.7%) | 1 (2.7%) | 10 (32.3%) | 5 (13.2%) | 11 (35.5%) | 9 (31.0%) | 0.002 |
| Loop diuretic (%) | 43 (25.9%) | 22 (59.5%) | 13 (41.9%) | 3 (7.9%) | 4 (12.9%) | 1 (3.4%) | <0.0001 |
| MRA (%) | 21 (12.4%) | 13 (35.1%) | 5 (16.1%) | 2 (5.3%) | 1 (3.2%) | 0 (0%) | <0.0001 |
| Statin (%) | 53 (31.9%) | 13 (35.1%) | 9 (29.0%) | 12 (31.6%) | 10 (32.3%) | 9 (31.0%) | 0.989 |
| Parameters of CPET | |||||||
| Peak O2 pulse (mL/beat) | 11.9 (9.64–14.89) | 11.04 (9.18–15.99) | 10.97 (7.78–13.76) | 12.16 (9.93–14.92) | 12.11 (9.42–15.1) | 12.12 (10.11–14.90) | 0.303 |
| Peak VO2/kg (mL/kg/min) | 22.9 (18.2–28.4) | 20.0 (15.9–26.0) | 21.3 (16.8–25.1) | 25.1 (19.1–29.7) | 23.4 (19.5–29.0) | 25.5 (19.4–31.9) | 0.045 |
| Peak VE (L/min) | 54.0 (43.0–65.0) | 60.0 (44.5–71.0) | 52.0 (37.0–63.0) | 59.0 (45.8–68.8) | 49.0 (41.0–60.5) | 49.0 (43.0–65.0) | 0.159 |
| AT (% of VO2 max) | 54.9 (45.8–66.2) | 50.0 (41.2–60.7) | 51.0 (45.7–57.8) | 58.2 (49.2–66.4) | 56.4 (44.6–73.5) | 61.7 (52.2–74.2) | 0.007 |
| VE/VCO2 at AT | 32.3 (29.2–35.8) | 33.4 (29.9–38.1) | 34.8 (29.8–37.9) | 31.7 (28.8–35.8) | 32.0 (28.9–34.1) | 30.9 (27.7–33.1) | 0.036 |
| Peak RER | 1.04 (0.98–1.09) | 1.05 (1.02–1.12) | 1.02 (0.97–1.09) | 1.05 (1.0–1.12) | 1.03 (0.96–1.07) | 1.04 (0.95–1.07) | 0.118 |
| ΔVO2/ΔWR (mL/min/W) | 11.6 (9.9–14.3) | 10.4 (8.1–12.6) | 11.2 (10.2–13.2) | 11.8 (9.9–14.4) | 11.4 (10.1–14.3) | 14.0 (10.8–16.0) | 0.015 |
| Peak VO2 (L/min) | 1600 (1233–2074) | 1528 (1101–2217) | 1461 (980–1676) | 1668 (1352–2114) | 1609 (1245–1982) | 1706 (1339–2117) | 0.152 |
| Peak Work (Watts) | 119.0 (77.5–161.5) | 135.0 (69.0–193.5) | 96.0 (74.0–125.0) | 125.5 (88.5–162.3) | 115.5 (79.8–158.5) | 123.0 (69.0–158.0) | 0.353 |
| Breathing Reserve (L) | 28.9 (15.1–42.0) | 34.0 (12.8–44.2) | 26.2 (10.6–40.0) | 30.9 (22.0–42.9) | 20.2 (8.5–35.6) | 33.2 (18.2–41.6) | 0.221 |
| (A) | ||||||||
| Variables | All Patients (n = 169) | LVEF ≤39% | LVEF 40–58% | LVEF 59–68% | LVEF 69–74% | LVEF ≥75% | p Value | |
| Primary endpoints | 49 (29%) | 20 (54.1%) | 10 (32.3%) | 8 (21.1%) | 6 (18.8%) | 5 (16.1%) | 0.002 | |
| Cardiovascular mortality | 18 (10.7%) | 10 (27.0%) | 5 (16.1%) | 2 (5.3%) | 0 (0%) | 1 (3.2%) | 0.001 | |
| (B) | ||||||||
| Variables | Non-HFpEF | HFpEF | p Value | Non-HFpEF with Ventilatory Inefficiency | HFpEF with Ventilatory Inefficiency | pValue | ||
| Primary endpoints | 27 (48.2%) | 22 (19.5%) | <0.0001 | 17 (58.6%) | 15 (51.7%) | 0.792 | ||
| Cardiovascular mortality | 12 (21.4%) | 6 (5.3%) | 0.001 | 9 (31.0%) | 5 (17.2%) | 0.358 | ||
| Independent Variable | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p Value | HR | (95% CI) | p Value | ||
| Age at CPET | 1.0 | (0.99–1.02) | 0.966 | ||||
| Male | 1.66 | (0.83–3.33) | 0.152 | ||||
| Lung Disease | |||||||
| Obstructive | 1.45 | (0.57–3.65) | 0.433 | ||||
| Restrictive | 1.73 | (0.99–3.02) | 0.057 | ||||
| Both | 1.92 | (1.09–3.40) | 0.025 | ||||
| Ischemic stroke | 1.56 | (0.56–4.44) | 0.392 | ||||
| Myocardial infarction | 1.31 | (0.66–2.63) | 0.442 | ||||
| Hypertension | 0.66 | (0.37–1.15) | 0.139 | ||||
| Prior PCI | 1.74 | (0.91–3.34) | 0.096 | ||||
| Diabetes | 2.06 | (1.14–3.71) | 0.016 | ||||
| Smoking | 1.97 | (1.10–3.56) | 0.024 | ||||
| LVEF | 0.97 | (0.96–0.98) | <0.001 | 0.98 | (0.96–0.99) | 0.002 | |
| Ischemic cardiomyopathy | 1.65 | (0.88–3.11) | 0.122 | ||||
| Dilated cardiomyopathy | 2.03 | (1.04–3.98) | 0.039 | ||||
| Valvular cardiomyopathy | 1.37 | (0.64–2.92) | 0.416 | ||||
| Beta-blocker | 2.24 | (1.19–4.22) | 0.013 | ||||
| ACEI/ARB | 1.88 | (0.96–3.69) | 0.064 | ||||
| DHP Ca+ channel blocker | 0.88 | (0.44–1.76) | 0.718 | ||||
| Loop diuretic | 3.39 | (1.93–5.96) | <0.001 | ||||
| MRA | 4.10 | (2.17–7.77) | <0.001 | ||||
| Statin | 1.57 | (0.89–2.78) | 0.121 | ||||
| VE/VCO2 at AT | 1.19 | (1.14–1.25) | <0.001 | 1.17 | (1.12–1.23) | <0.001 | |
| ΔVO2/ΔWR (mL/min/W) | 1.04 | (1.01–1.07) | 0.008 | ||||
| Peak O2 pulse (mL/beat) | 0.90 | (0.83–0.97) | 0.009 | ||||
| Peak VO2 (L/min) | 1.0 | (0.99–1.0) | 0.001 | ||||
| Peak RER | 0.27 | (0.01–5.60) | 0.395 | ||||
| Breathing reserve (mL) | 1.00 | (0.99–1.01) | 0.934 | ||||
| Peak VE (L/mins) | 1.0 | (0.98–1.01) | 0.731 | ||||
| Peak VO2/kg (mL/kg/mins) | 0.90 | (0.85–0.95) | <0.001 | ||||
| Peak work (Watts) | 0.99 | (0.99–1.0) | 0.009 | ||||
| Anaerobic threshold | 0.95 | (0.93–0.97) | <0.001 | ||||
| LVEF Group | VE/VCO2 at AT ≤34.3 | p Value | VE/VCO2 at AT >34.3 | p Value | All | p Value |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| ≤39 | 12.00 (1.50–96.01) | 0.019 | 1.63 (0.52–5.08) | 0.400 | 4.63 (1.74–12.35) | 0.002 |
| 40–58 | 3.49 (0.32–38.48) | 0.308 | 0.70 (0.21–2.33) | 0.561 | 2.12 (0.73–6.22) | 0.169 |
| 59–68 | 2.78 (0.29–26.74) | 0.376 | 0.63 (0.17–2.35) | 0.492 | 1.30 (0.42–3.97) | 0.647 |
| 69–74 | 2.92 (0.30–28.11) | 0.353 | 0.56 (0.12–2.50) | 0.445 | 1.12 (0.34–3.66) | 0.854 |
| ≥75 | 1 | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-M.; Wang, L.-Y.; Wu, P.-J.; Liaw, M.-Y.; Chen, Y.-L.; Chen, A.-N.; Tsai, T.-H.; Hang, C.-L.; Lin, M.-C. The Interrelationship between Ventilatory Inefficiency and Left Ventricular Ejection Fraction in Terms of Cardiovascular Outcomes in Heart Failure Outpatients. Diagnostics 2020, 10, 469. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics10070469
Chen S-M, Wang L-Y, Wu P-J, Liaw M-Y, Chen Y-L, Chen A-N, Tsai T-H, Hang C-L, Lin M-C. The Interrelationship between Ventilatory Inefficiency and Left Ventricular Ejection Fraction in Terms of Cardiovascular Outcomes in Heart Failure Outpatients. Diagnostics. 2020; 10(7):469. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics10070469
Chicago/Turabian StyleChen, Shyh-Ming, Lin-Yi Wang, Po-Jui Wu, Mei-Yun Liaw, Yung-Lung Chen, An-Ni Chen, Tzu-Hsien Tsai, Chi-Ling Hang, and Meng-Chih Lin. 2020. "The Interrelationship between Ventilatory Inefficiency and Left Ventricular Ejection Fraction in Terms of Cardiovascular Outcomes in Heart Failure Outpatients" Diagnostics 10, no. 7: 469. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics10070469