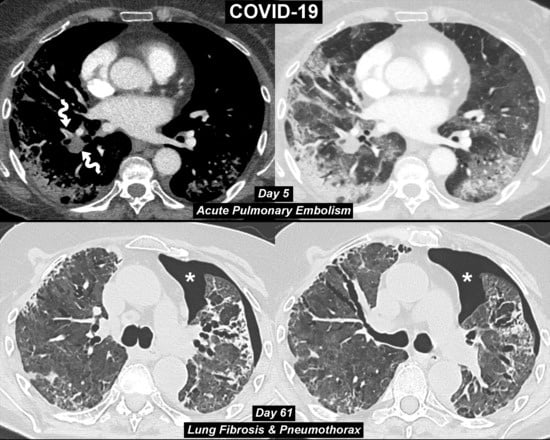
COVID-19 Pneumonia: Three Thoracic Complications in the Same Patient
Abstract
:Author Contributions
Funding
Conflicts of Interest
References
- Grillet, F.; Behr, J.; Calame, P.; Aubry, S.; Delabrousse, E. Acute Pulmonary Embolism Associated with COVID-19 Pneumonia Detected by Pulmonary CT Angiography. Radiology 2020. [Google Scholar] [CrossRef] [Green Version]
- Faggiano, P.; Bonelli, A.; Paris, S.; Milesi, G.; Bisegna, S.; Bernardi, N.; Curnis, A.; Agricola, E.; Maroldi, R. Acute pulmonary embolism in COVID-19 disease: Preliminary report on seven patients. Int. J. Cardiol. 2020. [Google Scholar] [CrossRef]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Casa, G.D.; Sverzellati, N.; Maher, T.M. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Gentile, F.; Aimo, A.; Forfori, F.; Catapano, G.; Clemente, A.; Cademartiri, F.; Emdin, M.; Giannoni, A. COVID-19 and risk of pulmonary fibrosis: The importance of planning ahead. Eur. J. Prev. Cardiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, K.; Drożdżal, S.; Machaj, F.; Rosik, J.; Szostak, B.; Zegan-Barańska, M.; Biernawska, J.; Dabrowski, W.; Rotter, I.; Kotfis, K. COVID-19: The Potential Treatment of Pulmonary Fibrosis Associated with SARS-CoV-2 Infection. J. Clin. Med. 2020, 9, 1917. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Onuki, T.; Ueda, S.; Yamaoka, M.; Sekiya, Y.; Yamada, H.; Kawakami, N.; Araki, Y.; Wakai, Y.; Saito, K.; Inagaki, M.; et al. Primary and Secondary Spontaneous Pneumothorax: Prevalence, Clinical Features, and In-Hospital Mortality. Can. Respir. J. 2017. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, R.J.; Goldacre, R.; Landray, M.J.; Rahman, N.M.; Goldacre, M.J. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968–2016. JAMA 2018, 320, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Zigliani, A.; Masciullo, R.; Golemi, S.; Maculotti, P.; Farina, D.; Maroldi, R. Radiographic severity index in COVID-19 pneumonia: Relationship to age and sex in 783 Italian patients. Radiol. Med. 2020, 125, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Borghesi, A.; Zigliani, A.; Golemi, S.; Carapella, N.; Maculotti, P.; Farina, D.; Maroldi, R. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: A study of 302 patients from Italy. Int. J. Infect Dis. 2020, 96, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, S.G.; Savietto, G.; Ballati, F.; Maggi, A.; Canino, C.; Bortolotto, C.; Valentini, A.; Dore, R.; Stella, G.M.; Corsico, A.G.; et al. Radiographic findings in 240 patients with COVID-19 pneumonia: Time-dependence after the onset of symptoms. Eur. Radiol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gervaise, A.; Bouzad, C.; Peroux, E.; Helissey, C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur. Radiol. 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Oliva, G. Acute pulmonary embolism in a patient with COVID-19 pneumonia. Diagn. Interv. Imaging 2020, 101, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Brogna, C.; Martino, A.; Minichiello, S.; Romeo, D.M.; Romano, P.; Bignardi, E.; Mazza, E.M.; Musto, L. SARS-CoV-2 Infection with Different Radiological Insights. Diagnostics 2020, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gao, R.; Zheng, Y.; Jiang, L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J. Travel Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ucpinar, B.A.; Sahin, C.; Yanc, U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: Case report. J. Infect Public Health 2020, 13, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Flower, L.; Carter, J.L.; Rosales Lopez, J.; Henry, A.M. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep. 2020, 13, e235861. [Google Scholar] [CrossRef] [PubMed]
- Spiro, J.E.; Sisovic, S.; Ockert, B.; Böcker, W.; Siebenbürger, G. Secondary tension pneumothorax in a COVID-19 pneumonia patient: A case report. Infection 2020, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghesi, A.; Aggiusti, C.; Farina, D.; Maroldi, R.; Muiesan, M.L. COVID-19 Pneumonia: Three Thoracic Complications in the Same Patient. Diagnostics 2020, 10, 498. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics10070498
Borghesi A, Aggiusti C, Farina D, Maroldi R, Muiesan ML. COVID-19 Pneumonia: Three Thoracic Complications in the Same Patient. Diagnostics. 2020; 10(7):498. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics10070498
Chicago/Turabian StyleBorghesi, Andrea, Carlo Aggiusti, Davide Farina, Roberto Maroldi, and Maria Lorenza Muiesan. 2020. "COVID-19 Pneumonia: Three Thoracic Complications in the Same Patient" Diagnostics 10, no. 7: 498. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics10070498