Association between Preoperative Retrograde Hepatic Vein Flow and Acute Kidney Injury after Cardiac Surgery †
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Definitions and Measurements (Variables and Data Sources and Grouping)
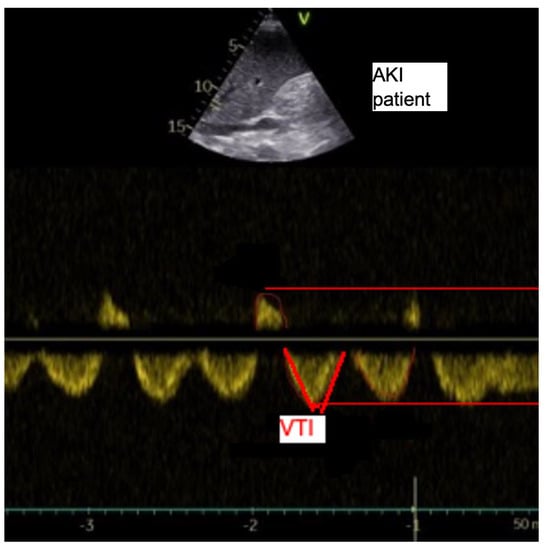
2.3. Analyses of the Hepatic Veins
2.4. Outcomes
2.5. Power Calculation
2.6. Statistical Analysis
3. Results
Descriptive Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petäjä, L.; Vaara, S.; Liuhanen, S.; Suojaranta-Ylinen, R.; Mildh, L.; Nisula, S.; Korhonen, A.-M.; Kaukonen, K.-M.; Salmenperä, M.; Pettilä, V. Acute Kidney Injury after Cardiac Surgery by Complete KDIGO Criteria Predicts Increased Mortality. J. Cardiothorac. Vasc. Anesth. 2017, 31, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyguła-Jurkiewicz, B.; Nadziakiewicz, P.; Zakliczynski, M.; Szczurek, W.; Chraponski, J.; Zembala, M.; Gasior, M. Predictive Value of Hepatic and Renal Dysfunction Based on the Models for End-Stage Liver Disease in Patients with Heart Failure Evaluated for Heart Transplant. Transplant. Proc. 2016, 48, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Seki, S.; Shiokawa, N.; Matsuba, T.; Miyazono, A.; Hazeki, D.; Imoto, Y.; Kawano, Y. Validation of acute kidney injury according to the modified KDIGO criteria in infants after cardiac surgery for congenital heart disease. Nephrology 2019, 24, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, V.; Kneidinger, N.; Herkner, H.; Heinz, G.; Nikfardjam, M.; Bojic, A.; Schellongowski, P.; Angermayr, B.; Kitzberger, R.; Warszawska, J.; et al. Hypoxic hepatitis: Underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med. 2009, 35, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Farr, M.; Mitchell, J.; Lippel, M.; Kato, T.S.; Jin, Z.; Ippolito, P.; Dove, L.; Jorde, U.P.; Takayama, H.; Emond, J.; et al. Combination of liver biopsy with MELD-XI scores for post-transplant outcome prediction in patients with advanced heart failure and suspected liver dysfunction. J. Heart Lung Transplant. 2015, 34, 873–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboelsoud, M.M.; Javaid, A.I.; Al-Qadi, M.O.; Lewis, J.H. Hypoxic hepatitis—Its biochemical profile, causes and risk factors of mortality in critically-ill patients: A cohort study of 565 patients. J. Crit. Care 2017, 41, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Birnie, K.; Verheyden, V.; Pagano, D.; Bhabra, M.; Tilling, K.; Sterne, J.A.; Murphy, G.J. Predictive models for kidney disease: Improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care 2014, 18, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carricart, M.; Denault, A.Y.; Couture, P.; Limoges, P.; Babin, D.; Levesque, S.; Fortier, A.; Pellerin, M.; Tardif, J.-C.; Buithieu, J. Incidence and significance of abnormal hepatic venous Doppler flow velocities before cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2005, 19, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Gorka, T.S.; Gorka, W. Doppler sonographic diagnosis of severe portal vein pulsatility in constrictive pericarditis: Flow normalization after pericardiectomy. J. Clin. Ultrasound. 1999, 27, 84–88. [Google Scholar] [CrossRef]
- Moller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R. European system for cardiac operative risk evaluation (EuroSCORE). Eur. J. Cardiothorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Yang, J.A.; Kato, T.S.; Shulman, B.P.; Takayama, H.; Farr, M.; Jorde, U.P.; Mancini, D.M.; Naka, Y.; Schulze, P.C. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the Model of End-stage Liver Disease (MELD) and MELD eXcluding INR (MELD-XI) scoring system. J. Heart Lung Transplant. 2012, 31, 601–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Yoshihisa, A.; Takiguchi, M.; Shimizu, T.; Nakamura, Y.; Yamauchi, H.; Iwaya, S.; Owada, T.; Miyata, M.; Sato, T.; et al. Liver dysfunction assessed by model for end-stage liver disease excluding INR (MELD-XI) scoring system predicts adverse prognosis in heart failure. PLoS ONE 2014, 9, e100618. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Navero, J.L.; Merino-Cejas, C.; de la Rosa, I.I.; Jaraba-Caballero, S.; Frias-Perez, M.; Gómez-Guzmán, E.; Gil-Campos, M.; de la Torre-Aguilar, M.J. Evaluation of the vasoactive-inotropic score, mid-regional pro-adrenomedullin and cardiac troponin I as predictors of low cardiac output syndrome in children after congenital heart disease surgery. Med. Intensiva 2019, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zwiebel, W.J. Sonographic diagnosis of hepatic vascular disorders. Semin. Ultrasound CT MR 1995, 16, 34–48. [Google Scholar] [CrossRef]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.-F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.-F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Sakoda, S.; Mitsunami, K.; Kinoshita, M. Evaluation of hepatic venous flow patterns using a pulsed Doppler technique. J. Cardiol. 1990, 20, 193–208. [Google Scholar]
- Maeda, K.; Murakami, A.; Takaoka, T.; Takamoto, S.; Sano, K.; Makuuchi, M. Usefulness of intraoperative color doppler ultrasonography in decision making regarding conversion of an accessory hepatic vein after a Fontan-type operation. Pediatr. Cardiol. 2004, 25, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Sauerheber, R.D.; Gordon, L.M.; Crosland, R.D.; Kuwahara, M.D. Spin-label studies on rat liver and heart plasma membranes: Do probe-probe interactions interfere with the measurement of membrane properties? J. Membr. Biol. 1977, 31, 131–169. [Google Scholar] [CrossRef]
- Scheinfeld, M.H.; Bilali, A.; Koenigsberg, M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics 2009, 29, 2081–2098. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.D.; Stafford-Smith, M.; White, W.D.; Phillips-Bute, B.; Swaminathan, M.; Milano, C.; Welsby, I.; Aronson, S.; Mathew, J.P.; Peterson, E.D.; et al. The effect of aprotinin on outcome after coronary-artery bypass grafting. N. Engl. J. Med. 2008, 358, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Arrigain, S.; Worley, S.; Yared, J.P.; Paganini, E.P. A clinical score to predict acute renal failure after cardiac surgery. J. Am. Soc. Nephrol. 2005, 16, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, M.N.; Nakazone, M.A.; Maia, L.N. Acute kidney injury based on KDIGO (Kidney Disease Improving Global Outcomes) criteria in patients with elevated baseline serum creatinine undergoing cardiac surgery. Rev. Bras. Cir. Cardiovasc. 2014, 29, 299–307. [Google Scholar] [CrossRef]
- Xue, H.; Li, C.; Cui, L.; Tian, C.; Li, S.; Wang, Z.; Ge, Q. M-BLUE protocol for coronavirus disease-19 (COVID-19) patients: Interobserver variability and correlation with disease severity. Clin. Radiol. 2021, 76, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaulitz, R.; Luhmer, I.; Kallfelz, H.C. Pulsed Doppler echocardiographic assessment of patterns of venous flow after the modified Fontan operation: Potential clinical implications. Cardiol. Young. 1998, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gardeback, M.; Settergren, G.; Brodin, L.A. Hepatic blood flow and right ventricular function during cardiac surgery assessed by transesophageal echocardiography. J. Cardiothorac. Vasc. Anesth. 1996, 10, 318–322. [Google Scholar] [CrossRef]
- Zieleskiewicz, L.; Lopez, A.; Hraiech, S.; Baumstarck, K.; Pastene, B.; Di Bisceglie, M.; Coiffard, B.; Duclos, G.; Boussuges, A.; Bobbia, X.; et al. Bedside POCUS during ward emergencies is associated with improved diagnosis and outcome: An observational, prospective, controlled study. Crit. Care 2021, 25, 34. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound. J. 2020, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Gagné, M.-P.; Richebé, P.; Loubert, C.; Drolet, P.; Gobert, Q.; Denault, A.; Zaphiratos, V. Ultrasound evaluation of inferior vena cava compression in tilted and supine term parturients. Can. J. Anaesth. 2021, 68, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Pettey, G.; Hermansen, J.L.; Nel, S.; Moutlana, H.J.; Muteba, M.; Juhl-Olsen, P.; Tsabedze, N.; Chakane, P.M. Ultrasound Hepatic Vein Ratios Are Associated with the Development of Acute Kidney Injury after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Caraba, A.; Iurciuc, S.; Munteanu, A.; Iurciuc, M. Hyponatremia and Renal Venous Congestion in Heart Failure Patients. Dis Markers 2021, 2021, 6499346. [Google Scholar] [CrossRef] [PubMed]
- Sandrikov, V.A.; Dzeranova, A.N.; Fedulova, S.V.; Lokshin, L.S.; Karshieva, A.R.; Kulagina, T.Y. Assessment of liver function with transesophageal echocardiography heart surgery with cardiopulmonary bypass. Anesteziol. Reanimatol. 2016, 61, 4–7. [Google Scholar] [PubMed]
- Hussain, A.; Via, G.; Melniker, L.; Goffi, A.; Tavazzi, G.; Neri, L.; Villen, T.; Hoppmann, R.; Mojoli, F.; Noble, V.; et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): International expert consensus. Crit. Care 2020, 24, 702. [Google Scholar] [CrossRef] [PubMed]
- Valentova, M.; von Haehling, S.; Doehner, W.; Murin, J.; Anker, S.D.; Sandek, A. Liver dysfunction and its nutritional implications in heart failure. Nutrition 2013, 29, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Okamoto, H.; Akiyoshi, K.; Irita, K.; Taniyama, T.; Takahashi, S. Effect of low-dose milrinone on gastric intramucosal pH and systemic inflammation after hypothermic cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2001, 15, 197–203. [Google Scholar] [CrossRef]
- Sugawara, Y.; Yoshihisa, A.; Ishibashi, S.; Matsuda, M.; Yamadera, Y.; Ohara, H.; Ichijo, Y.; Watanabe, K.; Hotsuki, Y.; Anzai, F.; et al. Liver Congestion Assessed by Hepatic Vein Waveforms in Patients with Heart Failure. CJC Open 2021, 3, 778–786. [Google Scholar] [CrossRef]
- Yokoyama, H.; Sekino, M.; Funaoka, H.; Sato, S.; Araki, H.; Egashira, T.; Yano, R.; Matsumoto, S.; Ichinomiya, T.; Higashijima, U.; et al. Association between enterocyte injury and fluid balance in patients with septic shock: A post hoc exploratory analysis of a prospective observational study. BMC Anesthesiol. 2021, 21, 293. [Google Scholar] [CrossRef]
- Uhlig, C.; Neto, A.S.; Van Der Woude, M.; Kiss, T.; Wittenstein, J.; Shelley, B.; Scholes, H.; Hiesmayr, M.; Melo, M.F.V.; Sances, D.; et al. Intraoperative mechanical ventilation practice in thoracic surgery patients and its association with postoperative pulmonary complications: Results of a multicenter prospective observational study. BMC Anesthesiol. 2020, 20, 179. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Beaubien-Souligny, W.; Oussaïd, E.; Henri, C.; Racine, N.; Denault, A.Y.; Rouleau, J.L. Assessing Splanchnic Compartment Using Portal Venous Doppler and Impact of Adding It to the EVEREST Score for Risk Assessment in Heart Failure. CJC Open 2020, 2, 311–320. [Google Scholar] [CrossRef] [PubMed]




| All Patients | AKI | Non-AKI | p | |
|---|---|---|---|---|
| Male | 66 (67%) | 11 (62%) | 55 (70%) | 0.43 |
| Female (Nr) | 32 (33%) | 6 (38%) | 26 (30%) | 0.16 |
| Age (years) | 68.8 (11.2) | 69.1 (7.4) | 63.5 (13.9) | 0.015 |
| Diabetes | 20 (20%) | 5 (25%) | 15 (19%) | 0.09 |
| NYHA III/IV | 41 (41%) | 10 (58.8%) | 31 (39%) | 0.12 |
| EuroSCORE | 1.6 (0.9) | 1.6 (1.0) | 1.5 (0.7) | 0.09 |
| Weight (kg) | 74.6 (8.1) | 72.6 (7.1) | 75.1 (9.12) | 0.43 |
| Operation time (min) | 182.4 (39.1) | 178.1 (41.1) | 188. 8 (39.1) | 0.88 |
| Aorta cross clamp time (min) | 47.8 (7.1) | 40.8 (9.1) | 48.1 (7.6) | 0.73 |
| Type of surgery | ||||
| AVR | 28 (29%) | 6 (35%) | 22 (26%) | 0.11 |
| CABG | 39 (40%) | 7 (41%) | 32 (39%) | 0.06 |
| MVR | 20 (20%) | 3 (17%) | 17 (20%) | 0.09 |
| Combined | 12 (12%) | 1 (7%) | 11 (15%) | 0.12 |
| Hemoglobin (g/L) | 137.1 (18.5) | 135.3 (19.7) | 138.4 (18.7) | 0.61 |
| Albumin (g/L) | 41.4 (8.6) | 38.8 (8.1) | 43.7 (7.9) | 0.83 |
| Platelets (1000/L) | 217.5 (62.2) | 218.6 (64.9) | 211.4 (58.7) | 0.75 |
| CRP (mg/L) | 4.3 (3.3) | 4.2 (3.9) | 4.5 (3.3) | 0.53 |
| INR | 1.9 (10.5) | 1.1 (0.3) | 3.9 (22.2) | 0.34 |
| ASAT (U/L) | 20.9 (10.4) | 18.8 (6.3) | 21.2 (13.2) | 0.34 |
| ALAT (U/L) | 31.4 (21.3) | 34.2 (18.9) | 30.4 (27.3) | 0.56 |
| Creatinine (µmol/L) | 87.8 (20.1) | 109.8 (25.1) | 78.4 (17.7) | 0.001 |
| Urea Nitrogen (mmol/L) | 6.4 (2.1) | 5.9 (1.8) | 7.4 (3.2) | 0.18 |
| GFR (ml/min/1.73 ) | 79.7 (15.4) | 80.2 (15.6) | 76.0 (16.6) | 0.004 |
| Bilirubin (µmol/L) | 17.8 (43.3) | 11.4 (7.4) | 21.8 (76.5) | 0.31 |
| MELD | 7.1 (19.0) | 7.50 (4.6) | 5.3 (1.2) | 0.50 |
| NYHA | 2.1 (0.5) | 2.1 (0.4) | 2.2 (0.5) | 0.10 |
| EuroSCORE | 1.6 (0.9) | 1.6 (0.9) | 1.5 (1.0) | 0.09 |
| Univariable Linear Regression | Multivariable Regression | |||||||
|---|---|---|---|---|---|---|---|---|
| B | 95% CI | p Value | B | 95% CI | p Value | |||
| Avmax (m/s) | 0.640 | 0.332 | 0.948 | <0.001 | 0.714 | 0.437 | 0.991 | <0.001 |
| A VTI (cm) | 0.035 | 0.021 | 0.050 | <0.001 | 0.038 | 0.025 | 0.051 | <0.001 |
| Svmax (m/s) | 0.049 | −0.153 | 0.251 | 0.631 | ||||
| S VTI (cm) | −0.002 | −0.011 | 0.007 | 0.691 | ||||
| Vvmax (m/s) | 0.075 | −0.127 | 0.277 | 0.462 | ||||
| V VTI (cm) | 0.011 | −0.003 | 0.025 | 0.127 | ||||
| Dvmax (m/s) | 0.151 | −0.163 | 0.466 | 0.342 | ||||
| D VTI (cm) | 0.010 | −0.005 | 0.024 | 0.177 | ||||
| Anterovmax (m/s) | 0.062 | −0.080 | 0.204 | 0.388 | ||||
| Retrovmax (m/s) | 0.168 | −0.009 | 0.345 | 0.062 | ||||
| Ratiovmax | 0.091 | −0.124 | 0.307 | 0.402 | ||||
| AnteroVTI (cm) | 0.001 | −0.006 | 0.009 | 0.690 | ||||
| RetroVTI (cm) | 0.017 | 0.006 | 0.027 | 0.002 | 0.018 | 0.008 | 0.027 | <0.001 |
| RatioVTI | 0.218 | 0.086 | 0.351 | 0.002 | 0.233 | 0.111 | 0.356 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eke, C.; Szabó, A.; Nagy, Á.; Párkányi, B.; Kertai, M.D.; Fazekas, L.; Kovács, A.; Lakatos, B.; Hartyánszky, I.; Gál, J.; et al. Association between Preoperative Retrograde Hepatic Vein Flow and Acute Kidney Injury after Cardiac Surgery. Diagnostics 2022, 12, 699. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics12030699
Eke C, Szabó A, Nagy Á, Párkányi B, Kertai MD, Fazekas L, Kovács A, Lakatos B, Hartyánszky I, Gál J, et al. Association between Preoperative Retrograde Hepatic Vein Flow and Acute Kidney Injury after Cardiac Surgery. Diagnostics. 2022; 12(3):699. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics12030699
Chicago/Turabian StyleEke, Csaba, András Szabó, Ádám Nagy, Boglár Párkányi, Miklós D. Kertai, Levente Fazekas, Attila Kovács, Bálint Lakatos, István Hartyánszky, János Gál, and et al. 2022. "Association between Preoperative Retrograde Hepatic Vein Flow and Acute Kidney Injury after Cardiac Surgery" Diagnostics 12, no. 3: 699. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics12030699