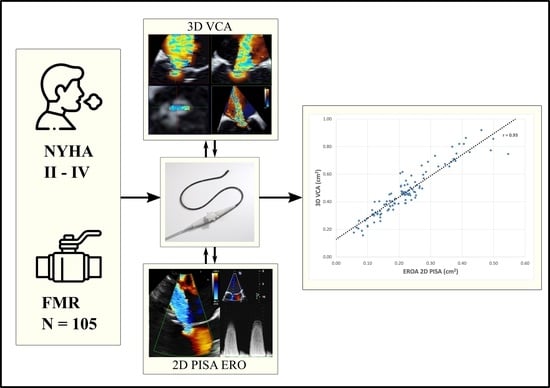
Benefit of 3D Vena Contracta Area over 2D-Based Echocardiographic Methods in Quantification of Functional Mitral Valve Regurgitation
Abstract
:1. Introduction
The Aims of This Study
- (1)
- Further substantiation of the measurement accuracy and reproducibility of 3D VCA in FMR;
- (2)
- Additional proof for reliability of 3D VCA in situations with eccentric or multiple regurgitation jets;
- (3)
- Determination of a cut-off value for 3D VCA to discriminate between moderate and severe FMR.
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Parameters
2.3. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
3.2. Echocardiographic Parameters
3.2.1. Correlations and Bland–Altman Analysis of 3D VCA
3.2.2. Threshold Determination for 3D VCA
3.2.3. Reproducibility of Measurements
4. Discussion
- (1)
- We demonstrated superior measurement accuracy and reproducibility of the 3D VCA compared to the 2D-based method in a collective, with a purely functional origin of mitral regurgitation;
- (2)
- We showed that the 3D VCA is a robust parameter in challenging situations in FMR, especially in eccentric or multiple regurgitation jets;
- (3)
- We established a cut-off value for the 3D VCA of 0.43 cm2 to differentiate between moderate and severe FMR, with high diagnostic quality.
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goliasch, G.; Bartko, P.E.; Pavo, N.; Neuhold, S.; Wurm, R.; Mascherbauer, J.; Lang, I.M.; Strunk, G.; Hülsmann, M. Refining the Prognostic Impact of Functional Mitral Regurgitation in Chronic Heart Failure. Eur. Heart J. 2018, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; On behalf of the Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the Echocardiographic Assessment of Native Valvular Regurgitation: An Executive Summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonow, R.O.; O’Gara Patrick, T.; Adams, D.H.; Badhwar, V.; Bavaria, J.E.; Elmariah, S.; Hung, J.W.; Lindenfeld, J.; Morris, A.A.; Satpathy, R.; et al. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation. J. Am. Coll. Cardiol. 2020, 75, 2236–2270. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, P.; Plicht, B.; Schenk, I.M.; Janosi, R.-A.; Erbel, R.; Buck, T. Direct Assessment of Size and Shape of Noncircular Vena Contracta Area in Functional Versus Organic Mitral Regurgitation Using Real-Time Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Ashikhmina, E.; Shook, D.; Cobey, F.; Bollen, B.; Fox, J.; Liu, X.; Worthington, A.; Song, P.; Shernan, S. Three-Dimensional Versus Two-Dimensional Echocardiographic Assessment of Functional Mitral Regurgitation Proximal Isovelocity Surface Area. Anesth. Analg. 2015, 120, 534–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goebel, B.; Heck, R.; Hamadanchi, A.; Otto, S.; Doenst, T.; Jung, C.; Lauten, A.; Figulla, H.R.; Schulze, P.C.; Poerner, T.C. Vena Contracta Area for Severity Grading in Functional and Degenerative Mitral Regurgitation: A Transoesophageal 3D Colour Doppler Analysis in 500 Patients. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Shanks, M.; Siebelink, H.-M.J.; Delgado, V.; van de Veire, N.R.L.; Ng, A.C.T.; Sieders, A.; Schuijf, J.D.; Lamb, H.J.; Ajmone Marsan, N.; Westenberg, J.J.M.; et al. Quantitative Assessment of Mitral Regurgitation: Comparison Between Three-Dimensional Transesophageal Echocardiography and Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2010, 3, 694–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, A.; Palka, P.; Donnelly, J.E.; Burstow, D.J. Quantification of Mitral Regurgitation Orifice Area by 3-Dimensional Echocardiography: Comparison with Effective Regurgitant Orifice Area by PISA Method and Proximal Regurgitant Jet Diameter. Int. J. Cardiol. 2002, 86, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Vengala, S.; Miller, A.P.; Nanda, N.C.; Lloyd, S.G.; Ahmed, S.; Sinha, A.; Mehmood, F.; Bodiwala, K.; Upendram, S.; et al. Quantification of Mitral Regurgitation by Live Three-Dimensional Transthoracic Echocardiographic Measurements of Vena Contracta Area. Echocardiography 2004, 21, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, E.; Iwata, S.; Tugcu, A.; Arai, K.; Shimada, K.; Muro, T.; Yoshikawa, J.; Yoshiyama, M.; Gillam, L.D.; Hahn, R.T.; et al. Direct Measurement of Multiple Vena Contracta Areas for Assessing the Severity of Mitral Regurgitation Using 3D TEE. JACC Cardiovasc. Imaging 2012, 5, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigioni, F.; Enriquez-Sarano, M.; Zehr, K.J.; Bailey, K.R.; Tajik, A.J. Ischemic Mitral Regurgitation: Long-Term Outcome and Prognostic Implications with Quantitative Doppler Assessment. Circulation 2001, 103, 1759–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, S.; Salgo, I.S.; Sugeng, L.; Weinert, L.; Settlemier, S.H.; Mor-Avi, V.; Lang, R.M. A Three-Dimensional Insight into the Complexity of Flow Convergence in Mitral Regurgitation: Adjunctive Benefit of Anatomic Regurgitant Orifice Area. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1015–H1024. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, K.; Ito, H.; Kawano, S.; Okamura, A.; Kurotobi, T.; Date, M.; Inoue, K.; Fujii, K. Comparison of Orifice Area by Transthoracic Three-Dimensional Doppler Echocardiography Versus Proximal Isovelocity Surface Area (PISA) Method for Assessment of Mitral Regurgitation. Am. J. Cardiol. 2006, 97, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Marsan, N.A.; Westenberg, J.J.M.; Ypenburg, C.; Delgado, V.; van Bommel, R.J.; Roes, S.D.; Nucifora, G.; van der Geest, R.J.; de Roos, A.; Reiber, J.C.; et al. Quantification of Functional Mitral Regurgitation by Real-Time 3D Echocardiography. JACC Cardiovasc. Imaging 2009, 2, 1245–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Levine, R.A.; Hua, L.; Morris, E.L.; Kang, Y.; Flaherty, M.; Morgan, N.V.; Hung, J. Diagnostic Value of Vena Contracta Area in the Quantification of Mitral Regurgitation Severity by Color Doppler 3D Echocardiography. Circ Cardiovasc Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Vandervoort, P.M.; Rivera, J.M.; Mele, D.; Palacios, I.F.; Dinsmore, R.E.; Weyman, A.E.; Levine, R.A.; Thomas, J.D. Application of Color Doppler Flow Mapping to Calculate Effective Regurgitant Orifice Area. An in Vitro Study and Initial Clinical Observations. Circulation 1993, 88, 1150–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altiok, E.; Hamada, S.; van Hall, S.; Hanenberg, M.; Dohmen, G.; Almalla, M.; Grabskaya, E.; Becker, M.; Marx, N.; Hoffmann, R. Comparison of Direct Planimetry of Mitral Valve Regurgitation Orifice Area by Three-Dimensional Transesophageal Echocardiography to Effective Regurgitant Orifice Area Obtained by Proximal Flow Convergence Method and Vena Contracta Area Determined by Color Doppler Echocardiography. Am. J. Cardiol. 2011, 107, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Cobey, F.C.; Ferreira, R.; Ursprung, W.W.; Karhausen, J.; Swaminathan, M.; Mackensen, G.B. A Novel Approach to Assess the Three-Dimensional Anatomy of a Mitral Valve Regurgitant Jet Orifice. J. Cardiothorac. Vasc. Anesth. 2017, 31, 169–173. [Google Scholar] [CrossRef]







| Characteristics | n = 105 |
|---|---|
| Clinical: | |
| Age, years | 79 [75; 83] |
| Male sex | 69 (66) |
| BMI, kg/m2 | 25.8 [23.5; 29.6] |
| NYHA class | |
| II | 31 (30) |
| III | 58 (55) |
| IV | 16 (15) |
| Systolic blood pressure, mmHg | 106 [95; 125] |
| Heart frequency, beats/minute | 76 [55; 85] |
| Etiology: | |
| Ischemic cardiomyopathy | 62 (59) |
| Non-ischemic cardiomyopathy | 37 (35) |
| Combined cardiomyopathy | 6 (6) |
| Echocardiography: | |
| LVEDV, mL | 157 [121; 198] |
| LVESV, mL | 101 [62; 130] |
| LVEF, % | 38 [25.5; 50.0] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungels, V.M.; Heidrich, F.M.; Pfluecke, C.; Linke, A.; Sveric, K.M. Benefit of 3D Vena Contracta Area over 2D-Based Echocardiographic Methods in Quantification of Functional Mitral Valve Regurgitation. Diagnostics 2023, 13, 1176. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics13061176
Jungels VM, Heidrich FM, Pfluecke C, Linke A, Sveric KM. Benefit of 3D Vena Contracta Area over 2D-Based Echocardiographic Methods in Quantification of Functional Mitral Valve Regurgitation. Diagnostics. 2023; 13(6):1176. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics13061176
Chicago/Turabian StyleJungels, Vinzenz M., Felix M. Heidrich, Christian Pfluecke, Axel Linke, and Krunoslav M. Sveric. 2023. "Benefit of 3D Vena Contracta Area over 2D-Based Echocardiographic Methods in Quantification of Functional Mitral Valve Regurgitation" Diagnostics 13, no. 6: 1176. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics13061176