Preterm Perinatal Hypoxia-Ischemia Does not Affect Somatosensory Evoked Potentials in Adult Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rodents and Ethics Approval
2.2. Perinatal Hypoxia-Ischemia (HI) Procedure
2.3. Housing
2.4. Adhesive Removal (‘Sticker Test’)
2.5. Sedation
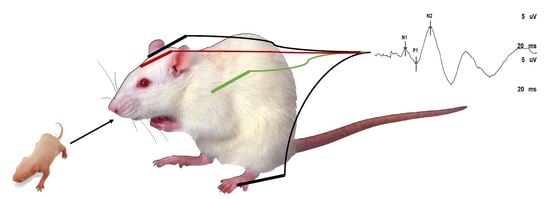
2.6. Somatosensory Evoked Potentials (SSEPs)
2.7. Statistics
3. Results
3.1. Adhesive Removal Test (‘Sticker Test’)
3.2. Evoked Potentials
4. Discussion
- (a)
- Due to our cross-sectional design, we might have missed SSEP changes in early postnatal age and adolescence.
- (b)
- Midazolam, ketamine and medetomidine were used as systemic anesthetics during SSEPs recordings. Accordingly, this anesthetic combination may have influenced SSEPs results; however, both experimental groups received the same anesthetic regime [23]. In humans, SSEPs are known to be less sensitive to the injectable anesthetics that we used, than to inhalation anesthetics [24].
- (c)
- The lumbar spinal cord apoptosis observed in severe HI models (19–20 min of submersion) may not necessarily be the postnatal hallmark of the moderate HI model (16–18 min of submersion) used in this experiment [21].
- (d)
- Evidence supports more neurological impairments and higher mortality for male preterm infants. In line with the human data, behavioral studies have consistently shown that the male sex is associated with an increased risk of long-term motor deficits in the rat preterm HIE [11]. Therefore, only male rats were included in this study.
- (e)
- Our global HI insult was conducted around the time of birth, when the rodent brain development is comparable to that of human infants born at a very low gestational age [11]. Studying SSEPs in larger animal models with brain development comparable to humans, such as sheep, may be more predictive of the human clinical situation [25]. However, larger animals are less suitable for chronic, long term studies.
- SSEPs are used to clinically assess the integrity of peripheral and central somatosensory pathways after perinatal HI.
- Childhood SSEPs are often used to predict long-term outcomes, but it is unknown if sensory pathway deficits persist into adulthood.
- We showed the electrophysiological and behavioral integrity of the somatosensory pathways in adult rats subjected to moderate perinatal HI.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CP | cerebral palsy |
| HI | hypoxia ischemia |
| SSEPs | somatosensory evoked potentials |
| HIE | hypoxic ischemic encephalopathy |
| ADHD | attention deficit/hyperactivity disorder |
| DCD | developmental coordination disorder |
References
- Trollmann, R.; Nüsken, E.; Wenzel, D. Neonatal Somatosensory Evoked Potentials: Maturational Aspects and Prognostic Value. Pediatr. Neurol. 2010, 42, 427–433. [Google Scholar] [CrossRef]
- Swarte, R.M.; Cherian, P.J.; Lequin, M.; Visser, G.H.; Govaert, P. Somatosensory evoked potentials are of additional prognostic value in certain patterns of brain injury in term birth asphyxia. Clin. Neurophysiol. 2012, 123, 1631–1638. [Google Scholar] [CrossRef]
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013, 74, 50–72. [Google Scholar] [CrossRef]
- Volpe, J.J. The neurological outcome of perinatal asphyxia. In Early Brain Damage V1: Research Orientations and Clinical Observations; Academic Press: Cambridge, MA, USA, 2012; p. 151. [Google Scholar]
- Nevalainen, P.; Marchi, V.; Metsäranta, M.; Lönnqvist, T.; Toiviainen-Salo, S.; Vanhatalo, S.; Lauronen, L. Evoked potentials recorded during routine EEG predict outcome after perinatal asphyxia. Clin. Neurophysiol. 2017, 128, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Garfinkle, J.; Sant’Anna, G.M.; Rosenblatt, B.; Majnemer, A.; Wintermark, P.; Shevell, M.I. Somatosensory evoked potentials in neonates with hypoxic-ischemic encephalopathy treated with hypothermia. Eur. J. Paediatr. Neurol. 2015, 19, 423–428. [Google Scholar] [CrossRef]
- Suppiej, A.; Cappellari, A.; Franzoi, M.; Traverso, A.; Ermani, M.; Zanardo, V. Bilateral loss of cortical somatosensory evoked potential at birth predicts cerebral palsy in term and near-term newborns. Early Hum. Dev. 2010, 86, 93–98. [Google Scholar] [CrossRef]
- Riquelme, I.; Montoya, P. Developmental changes in somatosensory processing in cerebral palsy and healthy individuals. Clin. Neurophysiol. 2010, 121, 1314–1320. [Google Scholar] [CrossRef]
- Teflioudi, E.P.; Zafeiriou, D.I.; Vargiami, E.; Kontopoulos, E.; Tsikoulas, I. Somatosensory Evoked Potentials in Children With Bilateral Spastic Cerebral Palsy. Pediatr. Neurol. 2011, 44, 177–182. [Google Scholar] [CrossRef]
- Zimmer, M.; Desch, L. Sensory Integration Therapies for Children with Developmental and Behavioral Disorders. Pediatrics 2012, 129, 1186–1189. [Google Scholar]
- Barkhuizen, M.; Hove, D.V.D.; Vles, J.; Steinbusch, H.; Kramer, B.; Gavilanes, A. 25 years of research on global asphyxia in the immature rat brain. Neurosci. Biobehav. Rev. 2017, 75, 166–182. [Google Scholar] [CrossRef]
- Strackx, E.; Hove, D.L.V.D.; Prickaerts, J.; Zimmermann, L.; Steinbusch, H.W.; Blanco, C.E.; Gavilanes, A.D.; Vles, J.H.; Gavilanes, A.W. Fetal asphyctic preconditioning protects against perinatal asphyxia-induced behavioral consequences in adulthood. Behav. Brain Res. 2010, 208, 343–351. [Google Scholar] [CrossRef]
- Loidl, C.; Gavilanes, A.; Van Dijk, E.H.; Vreuls, W.; Blokland, A.; Vles, J.S.; Steinbusch, H.W.; Blanco, C.E.; Gavilanes, A.W. Effects of hypothermia and gender on survival and behavior after perinatal asphyxia in rats. Physiol. Behav. 2000, 68, 263–269. [Google Scholar] [CrossRef]
- Barkhuizen, M.; Van Mechelen, R.; Vermeer, M.; Chedraui, P.; Paes, D.; Hove, D.L.V.D.; Vaes, B.; Mays, R.W.; Steinbusch, H.W.; Robertson, N.J.; et al. Systemic multipotent adult progenitor cells improve long-term neurodevelopmental outcomes after preterm hypoxic-ischemic encephalopathy. Behav. Brain Res. 2019, 362, 77–81. [Google Scholar] [CrossRef]
- Schallert, T.; Fleming, S.M.; Leasure, J.L.; Tillerson, J.L.; Bland, S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000, 39, 777–787. [Google Scholar] [CrossRef]
- Grow, J.L.; Liu, Y.Q.; Barks, J.D. Can Lateralizing Sensorimotor Deficits Be Identified after Neonatal Cerebral Hypoxia-Ischemia in Rats? Dev. Neurosci. 2003, 25, 394–402. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Huang, F.; Gates, M.; Holmberg, E.G. Somatosensory evoked potentials can be recorded on the midline of the skull with subdermal electrodes in non-sedated rats elicited by magnetic stimulation of the tibial nerve. J. Neurosci. Methods 2012, 208, 114–118. [Google Scholar] [CrossRef]
- Braddick, O.; Atkinson, J.; Innocenti, G. Perinatal brain damage in children: Neuroplasticity, early intervention, and molecular mechanisms of recovery. In Gene Expression to Neurobiology and Behaviour: Human Brain Development and Developmental Disorders; Elsevier: Amsterdam, The Netherlands, 2011; Volume 189, p. 139. [Google Scholar]
- Van Handel, M.; Swaab, H.; De Vries, L.S.; Jongmans, M.J. Long-term cognitive and behavioral consequences of neonatal encephalopathy following perinatal asphyxia: A review. Eur. J. Nucl. Med. Mol. Imaging 2007, 166, 645–654. [Google Scholar] [CrossRef]
- Hoon, A.H., Jr.; Stashinko, E.E.; Nagae, L.M.; Lin, D.D.; Keller, J.; Bastian, A.; Campbell, M.L.; Levey, E.; Mori, S.; Johnston, M.V. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev. Med. Child Neurol. 2009, 51, 697–704. [Google Scholar] [CrossRef] [Green Version]
- De Louw, A.; De Vente, J.; Steinbusch, H.; Gavilanes, A.W.; Steinbusch, H.; Blanco, C.; Troost, J.; Vles, J. Apoptosis in the rat spinal cord during postnatal development; the effect of perinatal asphyxia on programmed cell death. Neuroscience 2002, 112, 751–758. [Google Scholar] [CrossRef]
- Quairiaux, C.; Sizonenko, S.V.; Mégevand, P.; Michel, C.M.; Kiss, J.Z. Functional Deficit and Recovery of Developing Sensorimotor Networks following Neonatal Hypoxic-Ischemic Injury in the Rat. Cereb. Cortex 2010, 20, 2080–2091. [Google Scholar] [CrossRef] [Green Version]
- Hayton, S.M.; Kriss, A.; Muller, D.P. Comparison of the effects of four anaesthetic agents on somatosensory evoked potentials in the rat. Lab Anim. 1999, 33, 243–251. [Google Scholar] [CrossRef]
- Becker, A.; Amlong, C.; Rusy, D.A. Somatosensory-Evoked Potentials. In Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar]
- Anegroaie, P.; Frasch, M.; Rupprecht, S.; Antonow-Schlorke, I.; Müller, T.; Schubert, H.; Witte, O.; Schwab, M. Development of somatosensory-evoked potentials in foetal sheep: Effects of betamethasone. Acta Physiol. 2017, 220, 137–149. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkhuizen, M.; Vles, J.S.H.; van Mechelen, R.; Vermeer, M.; Kramer, B.W.; Chedraui, P.; Bergs, P.; van Kranen-Mastenbroek, V.H.J.M.; Gavilanes, A.W.D. Preterm Perinatal Hypoxia-Ischemia Does not Affect Somatosensory Evoked Potentials in Adult Rats. Diagnostics 2019, 9, 123. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9030123
Barkhuizen M, Vles JSH, van Mechelen R, Vermeer M, Kramer BW, Chedraui P, Bergs P, van Kranen-Mastenbroek VHJM, Gavilanes AWD. Preterm Perinatal Hypoxia-Ischemia Does not Affect Somatosensory Evoked Potentials in Adult Rats. Diagnostics. 2019; 9(3):123. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9030123
Chicago/Turabian StyleBarkhuizen, Melinda, Johan S.H. Vles, Ralph van Mechelen, Marijne Vermeer, Boris W. Kramer, Peter Chedraui, Paul Bergs, Vivianne H.J.M. van Kranen-Mastenbroek, and Antonio W.D. Gavilanes. 2019. "Preterm Perinatal Hypoxia-Ischemia Does not Affect Somatosensory Evoked Potentials in Adult Rats" Diagnostics 9, no. 3: 123. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9030123