Two Potential Clinical Applications of Origami-Based Paper Devices
Abstract
:1. Introduction
2. Materials and Methods
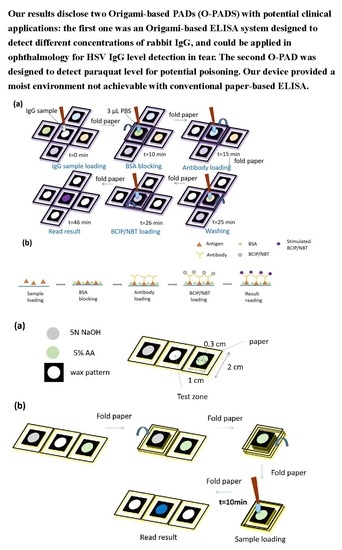
2.1. Design of Origami-Based PAD for ELISA
2.2. Performing Origami-Based PAD for ELISA
2.3. Fabrication of the Origami-Based PAD for Paraquat Detection
2.4. Performing Origami-Based Detection of Paraquat
3. Results and Discussion
3.1. O-PAD for ELISA
3.2. O-PAD for Paraquat Detection
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Y.H.; Kuo, Z.K.; Cheng, C.M. Paper—A potential platform in pharmaceutical development. Trends Biotechnol. 2015, 33, 4–9. [Google Scholar] [CrossRef]
- Sher, M.; Zhuang, R.; Demirci, U.; Asghar, W. Paper-based analytical devices for clinical diagnosis: Recent advances in the fabrication techniques and sensing mechanisms. Expert Rev. Mol. Diagn. 2017, 17, 351–366. [Google Scholar] [CrossRef]
- Cheng, C.M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based ELISA. Angew. Chem. Int. Ed. Engl. 2010, 49, 4771–4774. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.H.; Chen, K.H.; Hsu, M.Y.; Fan, S.T.; Huang, Y.F.; Chang, C.L.; Wang, Y.P.; Cheng, C.M. Evaluating organophosphate poisoning in human serum with paper. Talanta 2015, 144, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Hung, Y.C.; Hwang, D.K.; Lin, S.C.; Lin, K.H.; Wang, C.Y.; Choi, H.Y.; Wang, Y.P.; Cheng, C.M. Detection of aqueous VEGF concentrations before and after intravitreal injection of anti-VEGF antibody using low-volume sampling paper-based ELISA. Sci. Rep. 2016, 6, 34631. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Yang, C.Y.; Hsu, W.H.; Lin, K.H.; Wang, C.Y.; Shen, Y.C.; Chen, S.F.; Chau, S.F.; Tsai, H.Y.; Cheng, C.M. Monitoring the VEGF level in aqueous humor of patients with ophthalmologically relevant diseases via ultrahigh sensitive paper-based ELISA. Biomaterials 2014, 35, 3729–3735. [Google Scholar] [CrossRef]
- Yamada, K.; Takaki, S.; Komuro, N.; Suzuki, K.; Citterio, D. An antibody-free microfluidic paper-based analytical device for the determination of tear fluid lactoferrin by fluorescence sensitization of Tb3+. Analyst 2014, 139, 1637–1643. [Google Scholar] [CrossRef]
- Hsu, C.K.; Huang, H.Y.; Chen, W.R.; Nishie, W.; Ujiie, H.; Natzuga, K.; Fan, S.T.; Wang, H.K.; Lee, J.Y.Y.; Tsai, W.L.; et al. Paper-based ELISA for the detection of autoimmune antibodies in body fluid-the case of bullous pemphigoid. Anal. Chem. 2014, 86, 4605–4610. [Google Scholar] [CrossRef]
- Apilux, A.; Ukita, Y.; Chikae, M.; Chailapakul, O.; Takamura, Y. Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab Chip 2013, 13, 126–135. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef]
- Shih, C.M.; Chang, C.L.; Hsu, M.Y.; Lin, J.Y.; Kuan, C.M.; Wang, H.K.; Huang, C.T.; Chung, M.C.; Huang, K.C.; Hsu, C.E. Paper-based ELISA to rapidly detect Escherichia coli. Talanta 2015, 145, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Dungchai, W.; Chailapakul, O.; Henry, C.S. Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal. Chim Acta 2010, 674, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.M.; Lin, S.T.; Yen, T.H.; Wang, Y.L.; Cheng, C.M. Paper-based diagnostic devices for clinical paraquat poisoning diagnosis. Biomicrofluidics 2016, 10, 034118. [Google Scholar] [CrossRef] [PubMed]
- Barron, B.A.; Gee, L.; Hauck, W.W.; Kurinij, N.; Dawson, C.R.; Jones, D.B.; Wilhelmus, K.R.; Kaufman, H.E.; Sugar, J.; Hyndiuk, R.A. Herpetic Eye Disease Study: A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 1994, 101, 1871–1882. [Google Scholar] [CrossRef]
- Schwab, I.R.J.O. Oral acyclovir in the management of herpes simplex ocular infections. Ophthalmology 1988, 95, 423–430. [Google Scholar] [CrossRef]
- Bacon, A.S.; Dart, J.K.; Ficker, L.A.; Matheson, M.M.; Wright, P.J.O. Acanthamoeba keratitis: The value of early diagnosis. Virology 1993, 100, 1238–1243. [Google Scholar]
- German, A.; Hall, E.; Day, M.J. Measurement of IgG, IgM and IgA concentrations in canine serum, saliva, tears and bile. Vet. Immunol. 1998, 64, 107–121. [Google Scholar] [CrossRef]
- Bron, A.; Seal, D.J. The defences of the ocular surface. Trans. Ophthalmol. Soc. UK 1986, 105, 18–25. [Google Scholar]
- McClellan, B.H.; Whitney, C.R.; Newman, L.P.; Allansmith, M.R. Immunoglobulins in tears. Am. J. Ophthalmol. 1973, 76, 89–101. [Google Scholar] [CrossRef]
- Coyle, P.; Sibony, P.J. Tear immunoglobulins measured by ELISA. Investig. Ophth. Vis. Sci. 1986, 27, 622–625. [Google Scholar]
- Ashe, W.K.; Mage, M.; Notkins, A.L. Kinetics of neutralization of sensitized herpes simplex virus with antibody fragments. Short Commun. 1969, 37, 290–293. [Google Scholar] [CrossRef]
- Ashe, W.K.; Notkins, A.L. Kinetics of sensitization of herpes simplex virus and its relationship to the reduction in the neutralization rate constant. Virology 1967, 33, 613–617. [Google Scholar] [CrossRef]
- Centifanto, Y.; Kaufman, H.E. Secretory immunoglobulin A and herpes keratitis. Infect. Immun. 1970, 2, 778–782. [Google Scholar]
- Alizadeh, H.; Apte, S.; El-Agha, M.-S.H.; Li, L.; Hurt, M.; Howard, K.; Cavanagh, H.D.; McCulley, J.P.; Niederkorn, J.Y. Tear IgA and serum IgG antibodies against Acanthamoeba in patients with Acanthamoeba keratitis. Cornea 2001, 20, 622–627. [Google Scholar] [CrossRef]
- Wesseling, C.; De Joode, B.V.W.; Ruepert, C.; León, C.; Monge, P.; Hermosillo, H.; Partanen, L.J. Paraquat in developing countries. Int. J. Occup. Environ. Health 2001, 7, 275–286. [Google Scholar] [CrossRef]
- Pond, S.M.; Rivory, L.P.; Hampson, E.C.; Roberts, M.S. Kinetics of toxic doses of paraquat and the effects of hemoperfusion in the dog. J. Toxicol. Clin. Toxicol. 1993, 31, 229–246. [Google Scholar] [CrossRef]
- Sittipunt, C. Paraquat poisoning. Respir. Care. 2005, 50, 383–385. [Google Scholar]
- Weng, C.H.; Hu, C.C.; Lin, J.L.; Lin-Tan, D.T.; Hsu, C.W.; Yen, T.H. Predictors of acute respiratory distress syndrome in patients with paraquat intoxication. PLoS ONE 2013, 8, e82695. [Google Scholar] [CrossRef]
- Hsu, C.W.; Lin, J.L.; Lin.Tan, D.T.; Chen, K.H.; Yen, T.H.; Wu, M.S.; Lin, S.C. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PLoS ONE 2012, 7, e48397. [Google Scholar] [CrossRef]
- Johnson, M.; Chen, Y.; Hovet, S.; Xu, S.; Wood, B.; Ren, H.; Tokuda, J.; Tse, Z.T.H. Fabricating biomedical origami: A state-of-the-art review. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 2023–2032. [Google Scholar] [CrossRef]
- Liu, H.; Crooks, R.M. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem Soc. 2011, 133, 17564–17566. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, S.; Song, X.; Ge, S.; Yu, J. 3D origami-based multifunction-integrated immunodevice: Low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paper-based analytical device. Lab Chip 2012, 12, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, A.V.; Ramachandran, S.; Vigil, G.D.; Yager, P.; Bohringer, K.F. A low cost point-of-care viscous sample preparation device for molecular diagnosis in the developing world; an example of microfluidic origami. Lab Chip 2012, 12, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-A.; Yeh, W.-S.; Tsai, T.-T.; Chen, C.-F. Three-dimensional origami paper-based device for portable immunoassay applications. Lab Chip 2019, 19, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; An, Y.; Wu, Y.; Song, Y.; Zhu, Z.; Yang, C. Integrated Distance-Based Origami Paper Analytical Device for One-Step Visualized Analysis. ACS Appl. Mater. Interfaces 2017, 9, 30480–30487. [Google Scholar] [CrossRef]
- Chang, T.-H.; Tung, K.-H.; Gu, P.-W.; Yen, T.-H.; Cheng, C.-M. Rapid Simultaneous Determination of Paraquat and Creatinine in Human Serum Using a Piece of Paper. Micromachines 2018, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Donshik, P.C.; Ballow, M. Tear immunoglobulins in giant papillary conjunctivitis induced by contact lenses. Am. J. Ophthalmol. 1983, 96, 460–466. [Google Scholar] [CrossRef]
- Borderie, V.M.; Gineys, R.; Goldschmidt, P.; Batellier, L.; Laroche, L.; Chaumeil, C.J.C. Association of Anti–Herpes Simplex Virus IgG in Tears and Serum With Clinical Presentation in Patients With Presumed Herpetic Simplex Keratitis. Cornea 2012, 31, 1251–1256. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, C.; Martinez, A.; Mirica, K.; Li, X.; Phillips, S.; Mascarenas, M.; Whitesides, G. A portable microfluidic paper-based device for ELISA. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro. Mechanical Systems, Cancun, Mexico, 23–27 January 2011; pp. 75–78. [Google Scholar]
- Verma, M.S.; Tsaloglou, M.-N.; Sisley, T.; Christodouleas, D.; Chen, A.; Milette, J.; Whitesides, G.M. Sliding-strip microfluidic device enables ELISA on paper. Biosens Bioelectron. 2018, 99, 77–84. [Google Scholar] [CrossRef]
- Ramachandran, S.; Fu, E.; Lutz, B.; Yager, P.J.A. Long-term dry storage of an enzyme-based reagent system for ELISA in point-of-care devices. Analyst 2014, 139, 1456–1462. [Google Scholar] [CrossRef] [Green Version]





| Conventional | P-ELISA | Origami | |
|---|---|---|---|
| Pre-loaded reagents for assay | No | No | Yes |
| Reagents needed for conducting assay | Multiple reagents | Multiple reagents | One reagent or sample only |
| Equipment | Multiple tips | Multiple tips | Single tip |
| Convenience | Low | Low | High |
| Environment for reactions of detection | Less stable | Less stable | More stable |
| Required antigen volume | 70 μL | 3 μL | 2 μL |
| Total complete time | 213 min | 51 min | 46 min |
| LOD | 5.7 pM | 18 nM | 201 pM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Z.-K.; Chang, T.-H.; Chen, Y.-S.; Cheng, C.-M.; Tsai, C.-Y. Two Potential Clinical Applications of Origami-Based Paper Devices. Diagnostics 2019, 9, 203. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9040203
Kuo Z-K, Chang T-H, Chen Y-S, Cheng C-M, Tsai C-Y. Two Potential Clinical Applications of Origami-Based Paper Devices. Diagnostics. 2019; 9(4):203. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9040203
Chicago/Turabian StyleKuo, Zong-Keng, Tsui-Hsuan Chang, Yu-Shin Chen, Chao-Min Cheng, and Chia-Ying Tsai. 2019. "Two Potential Clinical Applications of Origami-Based Paper Devices" Diagnostics 9, no. 4: 203. https://0-doi-org.brum.beds.ac.uk/10.3390/diagnostics9040203