From Proteomics to Personalized Medicine: The Importance of Isoflavone Dose and Estrogen Receptor Status in Breast Cancer Cells
Abstract
:1. Introduction
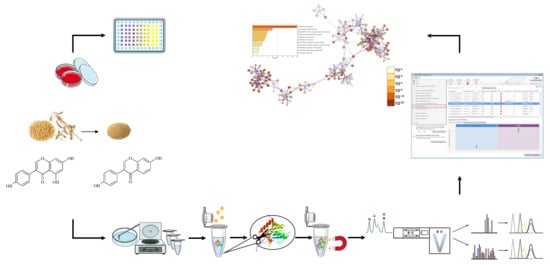
2. Materials and Methods
2.1. Cell Culture
2.2. MTT Assay
2.3. Sample Preparation for Proteomics Analysis
2.3.1. Protein Extraction
2.3.2. Protein Concentration Determination
2.3.3. Trypsin Digestion on Paramagnetic Beads
2.4. Protein Identification and Quantification by Nano-LC-UDMSE
2.5. Database Search
2.6. Statistical Analysis
2.7. Bioinformatics Analysis
3. Results and Discussion
3.1. Proteome Profiling by Data-Independent Nano-LC UDMSE Analysis
3.2. The Impact of Estrogen Receptor Status on Isoflavone Altered Pathways
3.3. The Impact of Isoflavone Dose on Protein Profile in MCF-7 Cells
4. Conclusions and Further Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dooijeweert, C.; Deckers, I.A.G.; Baas, I.O.; van der Wall, E.; van Diest, P.J. Hormone and HER2-receptor assessment in 33,046 breast cancer patients: A nationwide comparison of positivity rates between pathology laboratories in the Netherlands. Breast Cancer Res. Treat. 2019, 175, 487–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Uifălean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy isoflavones and breast cancer cell lines: Molecular mechanisms and future perspectives. Molecules 2015, 21, E13. [Google Scholar]
- Agrawal, G.K.; Timperio, A.M.; Zolla, L.; Bansal, V.; Shukla, R.; Rakwal, R. Biomarker discovery and applications for foods and beverages: Proteomics to nanoproteomics. J. Proteom. 2013, 93, 74–92. [Google Scholar] [CrossRef]
- Zhu, Z.; Edwards, R.J.; Boobis, A.R. Proteomic analysis of human breast cell lines using SELDI-TOF MS shows that mixtures of estrogenic compounds exhibit simple similar action (concentration additivity). Toxicol. Lett. 2008, 181, 93–103. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Q.; Wang, X.; Yang, X.; Wang, X.; Huang, Z.; Jiao, Y.; Wang, J. Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int. J. Oncol. 2016, 48, 1016–1028. [Google Scholar]
- Uifălean, A.; Schneider, S.; Gierok, P.; Ionescu, C.; Iuga, C.A.; Lalk, M. The impact of soy isoflavones on MCF-7 and MDA-MB-231 breast cancer cells using a global metabolomic approach. Int. J. Mol. Sci. 2016, 17, 1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uifălean, A.; Farcaş, A.; Ilieş, M.; Hegheş, S.C.; Ionescu, C.; Iuga, C.A. Assessment of isoflavone aglycones variability in soy food supplements using a validated HPLC-UV method. Clujul Med. 2015, 88, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, S.; Hentschker, C.; Nagel, A.; Hildebrandt, P.; Michalik, S.; Dittmar, D.; Surmann, K.; Völker, U. Improving proteome coverage for small sample amounts: An advanced method for proteomics approaches with low bacterial cell numbers. Proteomics 2019, 19, e1900192. [Google Scholar] [CrossRef] [Green Version]
- Ilies, M.; Iuga, C.A.; Loghin, F.; Dhople, V.M.; Thiele, T.; Völker, U.; Hammer, E. Impact of blood sample collection methods on blood protein profiling studies. Clin. Chim. Acta 2017, 471, 128–134. [Google Scholar]
- Distler, U.; Kuharev, J.; Navarro, P.; Tenzer, S. Label-free quantification in ion mobility–enhanced data-independent acquisition proteomics. Nat. Protoc. 2016, 11, 795–812. [Google Scholar] [CrossRef]
- Ilies, M.; Iuga, C.A.; Loghin, F.; Dhople, V.M.; Hammer, E. Plasma protein absolute quantification by nano-LC Q-TOF UDMSE for clinical biomarker verification. Med. Pharm. Rep. 2017, 90, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1–10. [Google Scholar]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131–3134. [Google Scholar] [CrossRef] [Green Version]
- Sotoca, A.M.; Ratman, D.; van der Saag, P.; Ström, A.; Gustafsson, J.A.; Vervoort, J.; Rietjens, I.M.C.M.; Murk, A.J. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERα/ERβ ratio. J. Steroid Biochem. Mol. Biol. 2008, 112, 171–178. [Google Scholar]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Fazel Nabavi, S.; Nabavi, M. Understanding genistein in cancer: The ‘‘good” and the ‘‘bad” effects: A review. Food Chem. 2015, 196, 589–600. [Google Scholar] [CrossRef]
- Lucki, N.C.; Sewer, M.B. Genistein stimulates MCF-7 breast cancer cell growth by inducing acid ceramidase (ASAH1) gene expression. J. Biol. Chem. 2011, 286, 19399–19409. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-Å. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Tsuboy, M.S.; Marcarini, J.C.; De Souza, A.O.; De Paula, N.A.; Dorta, D.J.; Mantovani, M.S.; Ribeiro, L.R. Genistein at maximal physiologic serum levels induces G0/G1 arrest in MCF-7 and HB4a cells, but not apoptosis. J. Med. Food 2014, 17, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajic, T.; Liu, Y.; Aebersold, R. Using data-independent, high-resolution mass spectrometry in protein biomarker research: Perspectives and clinical applications. Proteom. Clin. Appl. 2015, 9, 307–321. [Google Scholar] [CrossRef] [PubMed]
- QuickGO:Term GO:0016043. Available online: https://www.ebi.ac.uk/QuickGO/term/GO:0016043 (accessed on 2 November 2020).
- Sotoca, A.M.; Sollewijn Gelpke, M.D.; Boeren, S.; Ström, A.; Gustafsson, J.-Å.; Murk, A.J.; Rietjens, I.M.C.M.; Vervoort, J. Quantitative proteomics and transcriptomics addressing the estrogen receptor subtype-mediated effects in T47D breast cancer cells exposed to the phytoestrogen genistein. Mol. Cell. Proteom. 2011, 10, M110.002170. [Google Scholar]
- Engel, N.; Kraft, K.; Müller, P.; Duske, K.; Kühn, J.; Oppermann, C.; Nebe, B. Actin cytoskeleton reconstitution in MCF-7 breast cancer cells initiated by a native flax root extract. Adv. Med. Plant Res. 2015, 3, 92–105. [Google Scholar]
- Fioravanti, L.; Cappelletti, V.; Miodini, P.; Ronchi, E.; Brivio, M.; Di Fronzo, G. Genistein in the control of breast cancer cell growth: Insights into the mechanism of action in vitro. Cancer Lett. 1998, 130, 143–152. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, M.H.; Tzang, C.H.; Yang, M.; Wong, M.S. Inhibitory actions of genistein in human breast cancer (MCF-7) cells. Biochim. Biophys. Acta Mol. Basis Dis. 2003, 1638, 187–196. [Google Scholar]
- Rahal, O.M.; Simmen, R.C.M. PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis 2010, 31, 1491–1500. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.S.; Qi, W.T.; Guo, W.; Wang, C.L.; Hu, Z.B.; Li, A.K. Genistein and daidzein induce apoptosis of colon cancer cells by inhibiting the accumulation of lipid droplets. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Kousidou, O.C.; Mitropoulou, T.N.; Roussidis, A.E.; Kletsas, D.; Theocharis, A.D.; Karamanos, N.K. Genistein suppresses the invasive potential of human breast cancer cells through transcriptional regulation of metalloproteinases and their tissue inhibitors. Int. J. Oncol. 2005, 26, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; Allsopp, P.; Samaletdin, A.; Rowland, I.R. Daidzein, R-(+)equol and S-(-)equol inhibit the invasion of MDA-MB-231 breast cancer cells potentially via the down-regulation of matrix metalloproteinase-2. Eur. J. Nutr. 2014, 53, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Der Wang, S.; Chen, B.C.; Kao, S.T.; Liu, C.J.; Yeh, C.C. Genistein inhibits tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. BMC Complement. Altern. Med. 2014, 14, 26. [Google Scholar]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and breast cancer: Focus on angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sarkar, F.H. Gene expression profiles of genistein-treated PC3 prostate cancer cells. J. Nutr. 2002, 132, 3623–3631. [Google Scholar] [CrossRef]
- Chen, W.-F.; Wong, M.-S. Genistein enhances insulin-like growth factor signaling pathway in human breast cancer (MCF-7) cells. J. Clin. Endocrinol. Metab. 2004, 89, 2351–2359. [Google Scholar]
- Salminen, E.; Lagstro Èm, H.; Heikkila, È.S.; Salminen, S. Does breast cancer change patients’ dietary habits? Eur. J. Clin. Nutr. 2000, 54, 844–848. [Google Scholar] [CrossRef] [Green Version]
- Lei, Y.; Yeo, W.; Ho, S.C.; Lee, C.-K.I.; Cheung, K.L.; Lee, R.; He, Y.-Q. 207P Significant changes in dietary intake and physical activity after breast cancer diagnosis in a Chinese breast cancer cohort study. Ann. Oncol. 2017, 28 (Suppl. 5), 30. [Google Scholar] [CrossRef]
- Velentzis, L.S.; Brennan, S.F.; Woodside, J.V.; Keshtgar, M.R.; Leathem, A.J.; Titcomb, A.; Perkins, K.A.; Mazurowska, M.; Anderson, V.; Wardell, K.; et al. Significant changes in dietary intake and supplement use after breastcancer diagnosis in a UK prospective multicentre study. Proc. Nutr. Soc. 2010, 69, E378. [Google Scholar] [CrossRef] [Green Version]
- Baglia, M.L.; Zheng, W.; Li, H.; Yang, G.; Gao, J.; Gao, Y.-T.; Shu, X.-O. The association of soy food consumption with the risk of subtype of breast cancers defined by hormone receptor and HER2 status. Int. J. Cancer 2016, 139, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touillaud, M.; Gelot, A.; Mesrine, S.; Bennetau-Pelissero, C.; Clavel-Chapelon, F.; Arveux, P.; Bonnet, F.; Gunter, M.; Boutron-Ruault, M.C.; Fournier, A. Use of dietary supplements containing soy isoflavones and breast cancer risk among women aged >50 y: A prospective study. Am. J. Clin. Nutr. 2019, 109, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lv, J.; Guo, Y.; Bian, Z.; Gao, M.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; Wang, T.; et al. Soy intake and breast cancer risk: A prospective study of 300,000 Chinese women and a dose–response meta-analysis. Eur. J. Epidemiol. 2020, 35, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Test Compound | MCF-7 | MDA-MB-231 | |
|---|---|---|---|
| SC20 | IC20 | IC20 | |
| Gen (μM) | 5.62 | 22.44 | 11.04 |
| Dai (μM) | 19.01 | 52.24 | 36.39 |
| SSE (μg/mL) | 22.59 | 166.34 | 26.36 |
| MCF-7 Cells | MDA-MB-231 Cells | ||||||||
| UniProt | Protein Name | p Value | log2FC | UniProt | Protein Name | p Value | log2FC | ||
| Cellular Component Organization | Lipid Catabolism | ||||||||
| Microtubule Cytoskeleton Organization | Lipid Catabolic Process | ||||||||
| Gen IC20 | Q96N67 | Dedicator of cytokinesis protein 7 | 0.0342 | 5.06 | Gen IC20 | Q9NTX5 | Ethylmalonyl-CoA decarboxylase | 0.0015 | 7.97 |
| P16591 | Tyrosine-protein kinase Fer | 0.0007 | 4.98 | Q7Z5M8 | Abhydrolase domain containing 12B | 0.0000 | 4.85 | ||
| O43663 | Protein regulator of cytokinesis 1 | 0.0000 | 1.28 | Q9NY59 | Sphingomyelin phosphodiesterase 3 | 0.0067 | 1.67 | ||
| Cytoskeleton Dependent Cytokinesis | Phospholipid catabolic process | ||||||||
| Gen IC20 | P62745 | Rho-related GTP-binding protein RhoB | 0.0051 | −9.20 | Dai IC20 | Q13093 | Platelet-activating factor acetylhydrolase | 0.0022 | 9.76 |
| Q9NZ56 | Formin-2 | 0.0057 | −9.11 | Q7Z5M8 | Abhydrolase domain containing 12B | 0.0004 | 3.61 | ||
| O95630 | STAM-binding protein | 0.0150 | −1.21 | Q9NY59 | Sphingomyelin phosphodiesterase 3 | 0.0238 | 1.25 | ||
| Dai IC20 | P62745 | Rho-related GTP-binding protein RhoB | 0.0201 | −6.44 | Degradation of Extracellular Matrix | ||||
| Q9NZ56 | Formin-2 | 0.0013 | −6.34 | Gen IC20 | P42574 | Caspase-3 | 0.0002 | −2.95 | |
| Q13464 | Rho-associated protein kinase 1 | 0.0049 | −3.44 | P07858 | Cathepsin B | 0.0001 | −2.71 | ||
| Supramolecular fiber organization | Q13443 | Disintegrin and metalloproteinase domain-containing protein 9 | 0.0001 | −1.05 | |||||
| Gen IC20 | P62745 | Rho-related GTP-binding protein RhoB | 0.0051 | −9.20 | Dai IC20 | P42574 | Caspase-3 | 0.0034 | −2.43 |
| Q9NZ56 | Formin-2 | 0.0057 | −9.11 | O15230 | Laminin subunit alpha-5 | 0.0014 | −1.67 | ||
| P23258 | Tubulin gamma-1 chain | 0.0473 | −2.80 | P07711 | Cathepsin L1 | 0.0123 | −1.66 | ||
| SSE IC20 | P62745 | Rho-related GTP-binding protein RhoB | 0.0168 | −7.24 | SSE IC20 | P42574 | Caspase-3 | 0.0141 | −3.28 |
| Q9NZ56 | Formin-2 | 0.0075 | −7.14 | P07858 | Cathepsin B | 0.0049 | −2.53 | ||
| P23258 | Tubulin gamma-1 chain | 0.0207 | −6.03 | P09238 | Stromelysin-2 (Matrix metalloproteinase-10) | 0.0011 | −1.00 | ||
| Signaling by Receptor Tyrosine Kinases | mRNA Splicing | ||||||||
| Gen IC20 | P13942 | Collagen alpha-2(XI) chain | 0.0209 | 7.06 | 17S U2 snRNP | ||||
| Q96N67 P16591 | Dedicator of cytokinesis protein 7 Tyrosine-protein kinase Fer | 0.0342 0.0007 | 5.06 4.98 | Gen IC20 | P14678 | Small nuclear ribonucleoprotein-associated proteins B and B’ | 0.0000 | −2.44 | |
| P62318 | Small nuclear ribonucleoprotein Sm D3 | 0.0171 | −1.20 | ||||||
| Dai IC20 | P19388 | DNA-directed RNA polymerases I_ II_ and III subunit RPABC1 | 0.0031 | −8.48 | Q7L014 | Probable ATP-dependent RNA helicase DDX46 | 0.0004 | −1.16 | |
| Q9H6T0 | Epithelial splicing regulatory protein 2 | 0.0003 | −3.74 | RNA Polymerase II Transcription Termination | |||||
| Q13464 | Rho-associated protein kinase 1 | 0.0049 | −3.44 | SSE IC20 | P14678 | Small nuclear ribonucleoprotein-associated proteins B and B’ | 0.0034 | −2.60 | |
| Cell Cycle | P62318 | Small nuclear ribonucleoprotein Sm D3 | 0.0028 | −1.92 | |||||
| Gen SC20 | P23258 | Tubulin gamma-1 chain | 0.0396 | −11.85 | P26368 | Splicing factor U2AF 65 kDa subunit | 0.0003 | −1.54 | |
| P50402 | Emerin | 0.0437 | −11.60 | ||||||
| P19388 | DNA-directed RNA polymerases I_ II_ and III subunit RPABC1 | 0.0472 | −11.12 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilieș, M.; Uifălean, A.; Pașca, S.; Dhople, V.M.; Lalk, M.; Iuga, C.A.; Hammer, E. From Proteomics to Personalized Medicine: The Importance of Isoflavone Dose and Estrogen Receptor Status in Breast Cancer Cells. J. Pers. Med. 2020, 10, 292. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm10040292
Ilieș M, Uifălean A, Pașca S, Dhople VM, Lalk M, Iuga CA, Hammer E. From Proteomics to Personalized Medicine: The Importance of Isoflavone Dose and Estrogen Receptor Status in Breast Cancer Cells. Journal of Personalized Medicine. 2020; 10(4):292. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm10040292
Chicago/Turabian StyleIlieș, Maria, Alina Uifălean, Sergiu Pașca, Vishnu Mukund Dhople, Michael Lalk, Cristina Adela Iuga, and Elke Hammer. 2020. "From Proteomics to Personalized Medicine: The Importance of Isoflavone Dose and Estrogen Receptor Status in Breast Cancer Cells" Journal of Personalized Medicine 10, no. 4: 292. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm10040292