Vav1 Selectively Down-Regulates Akt2 through miR-29b in Certain Breast Tumors with Triple Negative Phenotype
Abstract
:1. Introduction
2. Materials and Methods
- Cell lines
- Modulation of Vav1 and miR-29b expression
- Animal models
- Reverse transcription quantitative PCR (RT-qPCR)
- Laser-capture micro-dissection and analysis of miR-29b expression
- Immunochemical analysis
- Immunohistochemical analysis
- Real-time cell invasion assays
- Statistical analysis
3. Results
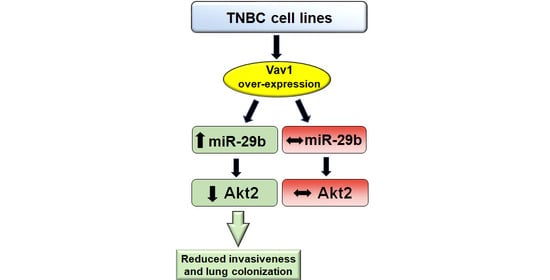
3.1. Vav1 Regulates Akt2 through miR-29b in MDA-MB-231 Cells
3.2. Vav1 Sustains the miR-29b/Akt2 Axis in MDA-MB-231 Cells In Vivo
3.3. Vav1 Regulates the miR-29b/Akt2 Axis in Cells from Patient-Derived Xenografts
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Jiang, Y.-Z.; Xu, X.-E.; Yu, K.-D.; Jin, X.; Hu, X.; Zuo, W.-J.; Hao, S.; Wu, J.; Liu, G.-Y. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res. 2016, 18, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef]
- Cserni, G.; Quinn, C.M.; Foschini, M.P.; Bianchi, S.; Callagy, G.; Chmielik, E.; Decker, T.; Fend, F.; Kovács, A.; van Diest, P.J.; et al. European Working Group For Breast Screening Pathology. Triple-Negative Breast Cancer Histological Subtypes with a Favourable Prognosis. Cancers 2021, 13, 5694. [Google Scholar] [CrossRef]
- Nath, A.; Mitra, S.; Mistry, T.; Pal, R.; Nasare, V.D. Molecular targets and therapeutics in chemoresistance of triple-negative breast cancer. Med. Oncol. 2021, 39, 14. [Google Scholar] [CrossRef]
- Rocca, P.A.C.; Orrù, S.; Muroni, M.R.; Sanges, F.; Sotgiu, G.; Ena, S.; Pira, G.; Murgia, L.; Manca, A.; Uras, M.G.; et al. Analysis of PIK3CA Mutations and Activation Pathways in Triple Negative Breast Cancer. PLoS ONE 2015, 10, e0141763. [Google Scholar]
- Pascual, J.; Turner, N.C. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann. Oncol. 2019, 30, 1051–1060. [Google Scholar] [CrossRef] [Green Version]
- Riggio, M.; Perrone, M.C.; Polo, M.L.; Rodriguez, M.J.; May, M.; Abba, M.; Lanari, C.; Novaro, V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci. Rep. 2017, 7, 44244. [Google Scholar] [CrossRef] [Green Version]
- Hinz, N.; Jücker, M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Commun. Signal. 2019, 17, 154. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Lambring, C.B. Akt Isoforms: A Family Affair in Breast Cancer. Cancers 2021, 13, 3445. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Chow, Z.; Lee, E.; Weiss, H.L.; Evers, B.M.; Rychahou, P. Role of AMPK and Akt in triple negative breast cancer lung colonization. Neoplasia 2021, 23, 429–438. [Google Scholar] [CrossRef]
- Rafael, D.; Gener, P.; Andrade, F.; Seras-Franzoso, J.; Montero, S.; Fernandez, Y.; Hidalgo, M.; Arango, D.; Sayos, J.; Florindo, H.F.; et al. AKT2 siRNA delivery with amphiphilic-based polymeric micelles show efficacy against cancer stem cells. Drug Deliv. 2018, 25, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Gener, P.; Rafael, D.; Seras-Franzoso, J.; Perez, A.; Pindado, L.A.; Casas, G.; Arango, D.; Fernandez, Y.; Diaz-Riascos, Z.V.; Abasolo, I.; et al. Pivotal Role of AKT2 during Dynamic Phenotypic Change of Breast Cancer Stem Cells. Cancers 2019, 11, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, B.; Paddar, M.; Khan, S.; Mir, S.A.; Clarke, P.; Grabowska, A.M.; Vijay, D.G.; Malik, F. AKT isoforms have discrete expression in triple negative breast cancers and roles in cisplatin sensitivity. Oncotarget 2020, 11, 4178–4194. [Google Scholar] [CrossRef]
- Tybulewicz, V.L. Vav-family proteins in T-cell signalling. Curr. Opin. Immunol. 2005, 17, 267–274. [Google Scholar] [CrossRef]
- Bertagnolo, V.; Brugnoli, F.; Grassilli, S.; Nika, E.; Capitani, S. Vav1 in differentiation of tumoral promyelocytes. Cell Signal 2012, 24, 612–620. [Google Scholar] [CrossRef]
- Vezzali, F.; Grassilli, S.; Lambertini, E.; Brugnoli, F.; Patergnani, S.; Nika, E.; Piva, R.; Pinton, P.; Capitani, S.; Bertagnolo, V. Vav1 is necessary for PU.1 mediated upmodulation of miR-29b in acute myeloid leukaemia-derived cells. J. Cell Mol. Med. 2018, 22, 3149–3158. [Google Scholar] [CrossRef] [Green Version]
- Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Rossi, C.; Perracchio, L.; Mottolese, M.; Marchisio, M.; Palomba, M.; Nika, E.; Natali, P.G.; et al. High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: A role in modulating genes related to the efficiency of metastatic process. Oncotarget 2014, 5, 4320–4336. [Google Scholar] [CrossRef] [Green Version]
- Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Marchisio, M.; Perracchio, L.; Piantelli, M.; Bavelloni, A.; Capitani, S.; Bertagnolo, V. Vav1 downmodulates Akt in different breast cancer subtypes: A new promising chance to improve breast cancer outcome. Mol. Oncol. 2018, 12, 1012–1025. [Google Scholar] [CrossRef] [Green Version]
- Honardoost, M.; Rad, S.M.A.H. Triangle of AKT2, miRNA, and Tumorigenesis in Different Cancers. Appl. Biochem. Biotechnol. 2018, 185, 524–540. [Google Scholar] [CrossRef]
- Bai, Y.; Li, J.; Li, J.; Liu, Y.; Zhang, B. MiR-615 inhibited cell proliferation and cell cycle of human breast cancer cells by suppressing of AKT2 expression. Int. J. Clin. Exp. Med. 2015, 8, 3801–3808. [Google Scholar] [PubMed]
- Pan, D.; Du, Y.; Li, R.; Shen, A.; Liu, X.; Li, C.; Hu, B. miR-29b-3p Increases Radiosensitivity in Stemness Cancer Cells via Modulating Oncogenes Axis. Front. Cell Dev. Biol. 2021, 9, 741074. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, S.; Vezzali, F.; Cairo, S.; Brugnoli, F.; Volinia, S.; De Mattei, M.; Judde, J.G.; Bertagnolo, V. Targeting the Vav1/miR-29b axis as a potential approach for treating selected molecular subtypes of triple-negative breast cancer. Oncol. Rep. 2021, 45, 83. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolo, V.; Grassilli, S.; Volinia, S.; Al-Qassab, Y.; Brugnoli, F.; Vezzali, F.; Lambertini, E.; Palomba, M.; Piubello, Q.; Orvieto, E.; et al. Ectopic expression of PLC-β2 in non-invasive breast tumor cells plays a protective role against malignant progression and is correlated with the deregulation of miR-146a. Mol. Carcinog. 2019, 58, 708–721. [Google Scholar] [CrossRef]
- Al-Qassab, Y.; Grassilli, S.; Brugnoli, F.; Vezzali, F.; Capitani, S.; Bertagnolo, V. Protective role of all-trans retinoic acid (ATRA) against hypoxia-induced malignant potential of non-invasive breast tumor derived cells. BMC Cancer 2018, 18, 1194. [Google Scholar] [CrossRef]
- Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Buglioni, S.; Bertagnolo, V. Vav1 Down-Modulates Akt2 Expression in Cells from Pancreatic Ductal Adenocarcinoma: Nuclear Vav1 as a Potential Regulator of Akt Related Malignancy in Pancreatic Cancer. Biomedicines 2020, 26, 379. [Google Scholar] [CrossRef]
- Brugnoli, F.; Grassilli, S.; Al-Qassab, Y.; Capitani, S.; Bertagnolo, V. PLC-β2 is modulated by low oxygen availability in breast tumor cells and plays a phenotype dependent role in their hypoxia-related malignant potential. Mol. Carcinog. 2016, 55, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Gjerstorff, M.F.; Benoit, V.M.; Laenkholm, A.V.; Nielsen, O.; Johansen, L.E.; Ditzel, H.J. Identification of genes with altered expression in medullary breast cancer vs. ductal breast cancer and normal breast epithelia. Int. J. Oncol. 2006, 28, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.; Martin, T.A.; Mansel, R.E.; Jiang, W.G. The expression and prognostic value of the guanine nucleotide exchange factors (GEFs) Trio, Vav1 and TIAM-1 in human breast cancer. Int. Semin. Surg. Oncol. 2008, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Sebban, S.; Farago, M.; Gashai, D.; Ilan, L.; Pikarsky, E.; Ben-Porath, I.; Katzav, S. Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PLoS ONE 2013, 8, e54321. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, S.; Nika, E.; Lambertini, E.; Brugnoli, F.; Piva, R.; Capitani, S.; Bertagnolo, V. A network including PU.1, Vav1 and miR-142-3p sustains ATRA-induced differentiation of acute promyelocytic leukemia cells—A short report. Cell Oncol. (Dordr) 2016, 39, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Wiechmann, S.; Ruprecht, B.; Siekmann, T.; Zheng, R.; Frejno, M.; Kunold, E.; Bajaj, T.; Zolg, D.P.; Sieber, S.A.; Gassen, N.V.; et al. Chemical Phosphoproteomics Sheds New Light on the Targets and Modes of Action of AKT Inhibitors. ACS Chem. Biol. 2021, 16, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Zhang, Y.; Qu, K.; Yang, X.; Fu, J.; Chen, W.; Li, X. MicroRNA-29B (mir-29b) regulates the Warburg effect in ovarian cancer by targeting AKT2 and AKT3. Oncotarget 2015, 6, 40799–40814. [Google Scholar] [CrossRef] [Green Version]
- Ibragimova, M.K.; Tsyganov, M.M.; Litviakov, N.V. Molecular-Genetic Portrait of Breast Cancer with Triple Negative Phenotype. Cancers 2021, 13, 5348. [Google Scholar] [CrossRef]
- Eyholzer, M.; Schmid, S.; Wilkens, L.; Mueller, B.U.; Pabst, T. The tumour-suppressive miR-29a/b1 cluster is regulated by CEBPA and blocked in human AML. Br. J. Cancer 2010, 103, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Ichavarria, I.; López-Tarruella, S.; Picornell, A.; García-Saenz, J.A.; Jerez, Y.; Hoadley, K.; Gómez, H.L.; Moreno, F.; Del Monte-Millan, M.; Márquez-Rodas, I.; et al. Pathological Response in a Triple-Negative Breast Cancer Cohort Treated with Neoadjuvant Carboplatin and Docetaxel According to Lehmann’s Refined Classification. Clin. Cancer Res. 2018, 24, 1845–1852. [Google Scholar] [CrossRef] [Green Version]
- Coussy, F.; de Koning, L.; Lavigne, M.; Bernard, V.; Ouine, B.; Boulai, A.; El Botty, R.; Dahmani, A.; Montaudon, E.; Assayag, F.; et al. A large collection of integrated genomically characterized patient-derived xenografts highlighting the heterogeneity of triple-negative breast cancer. Int. J. Cancer 2019, 145, 1902–1912. [Google Scholar] [CrossRef]
- Bou Zerdan, M.; Ghorayeb, T.; Saliba, F.; Allam, S.; Bou Zerdan, M.; Yaghi, M.; Bilani, N.; Jaafar, R.; Nahleh, Z. Triple Negative Breast Cancer: Updates on Classification and Treatment in 2021. Cancers 2022, 14, 1253. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassilli, S.; Brugnoli, F.; Cairo, S.; Bianchi, N.; Judde, J.-G.; Bertagnolo, V. Vav1 Selectively Down-Regulates Akt2 through miR-29b in Certain Breast Tumors with Triple Negative Phenotype. J. Pers. Med. 2022, 12, 993. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12060993
Grassilli S, Brugnoli F, Cairo S, Bianchi N, Judde J-G, Bertagnolo V. Vav1 Selectively Down-Regulates Akt2 through miR-29b in Certain Breast Tumors with Triple Negative Phenotype. Journal of Personalized Medicine. 2022; 12(6):993. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12060993
Chicago/Turabian StyleGrassilli, Silvia, Federica Brugnoli, Stefano Cairo, Nicoletta Bianchi, Jean-Gabriel Judde, and Valeria Bertagnolo. 2022. "Vav1 Selectively Down-Regulates Akt2 through miR-29b in Certain Breast Tumors with Triple Negative Phenotype" Journal of Personalized Medicine 12, no. 6: 993. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm12060993