A Proposal to Differentiate ACO, Asthma and COPD in Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Questionnaire
2.3. Lung Function and Allergy Evaluation
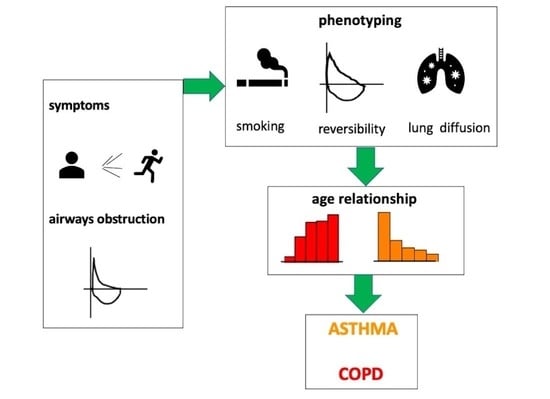
2.4. Spirometric Definitions (Figure 1)

- a “responder” was a post-BD FEV1/FVC ≥ LLN or an increase of FEV1 ≥ 200 mL and ≥ 12%. Among them:
- ○
- a “full responder” was a “responder” with a post BD FEV1 > 80% PV.
- ○
- an “incomplete responder” was a “responder” with a post BD FEV1 < 80% PV.
- an “irreversible” was a patient with a post BD response inducing a FEV1/FVC ≤ LLN and no significant increase of FEV1.
2.5. Statistics
3. Results
3.1. Definitions of 5 Phenotypes
3.2. The Relative Prevalence of COPD and Asthma during the Lifespan
3.3. The Relationship between Age and the Prevalence of the 5 Phenotypes
3.4. The Relationship between Age and the Prevalence of ACO, in Regard to Diffusion Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Bateman, E.D.; Reddel, H.K.; van Zyl-Smit, R.N.; Agusti, A. The asthma-COPD overlap syndrome: Towards a revised taxonomy of chronic airways diseases? Lancet Respir. Med. 2015, 3, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Postma, D.S.; Rabe, K.F. The asthma-COPD overlap syndrome. N. Engl. J. Med. 2015, 373, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.J. Asthma-COPD overlap. Chest 2016, 149, 7–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, J.M.; Sin, D.D. Asthma-COPD overlap syndrome: Pathogenesis, clinical features, and therapeutic targets. BMJ 2017, 358, j3772. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Wong, G.W. Changing trends and challenges in the management of asthma in Asia. J. Allergy Clin. Immunol. 2017, 140, 1272–1274. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.T.; Godin, I.; Phuong, N.T.; Nguyen, L.H.; Hiep, T.T.M.; Michel, O. Allergen sensitization among chronic respiratory diseases in urban and rural areas of the South of Vietnam. Int. J. Tuberc. Lung Dis. 2018, 22, 221–229. [Google Scholar] [CrossRef]
- Tran, D.; Kosik, R.; Mandell, G.; Chen, Y.; Su, T.; Chiu, A.; Fan, A. Tobacco control in Vietnam. Public Health 2013, 127, 109–118. [Google Scholar] [CrossRef]
- Postma, D.S.; Weiss, S.T.; van den Berge, M.; Kerstjens, H.A.; Koppelman, G.H. Revisiting the Dutch hypothesis. J. Allergy Clin. Immunol. 2015, 136, 521–529. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, C.; Peto, R.; Tinker, C.; Speizer, F.E. The Natural History of Chronic Bronchitis and Emphysema. An Eight-Year Study of Early Chronic Obstructive Lung Disease in Working Men in London; Oxford University Press: London, UK, 1976. [Google Scholar]
- de Vries, M.; Faiz, A.; Woldhuis, R.R.; Postma, D.S.; de Jong, T.V.; Sin, D.D.; Bossé, Y.; Nickle, D.C.; Guryev, V.; Timens, W.; et al. Lung tissue gene-expression signature for the ageing lung in COPD. Thorax 2018, 73, 609–617. [Google Scholar] [CrossRef]
- Duong-Quy, S.; Van, H.T.; Kim, A.V.T.; Huy, Q.P.; Craig, T.J. Clinical and Functional Characteristics of Subjects with Asthma, COPD, and Asthma-COPD Overlap: A Multicentre Study in Vietnam. Can. Respir. J. 2018, 2018, 1732946. [Google Scholar] [CrossRef]
- Global Strategy for Asthma Management and Prevention. 2022. Available online: http://www.ginasthma.org (accessed on 14 November 2022).
- Sin, D.D.; Tashkin, D.; Zhang, X.; Radner, F.; Sjöbring, U.; Thorén, A.; Calverley, P.M.; I Rennard, S. Budesonide and the risk of pneumonia: A meta-analysis of individual patient data. Lancet 2009, 374, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Crim, C.; Dransfield, M.T.; Bourbeau, J.; Jones, P.W.; Hanania, N.A.; Mahler, D.A.; Vestbo, J.; Wachtel, A.; Martinez, F.J.; Barnhart, F.; et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann. Am. Thorac. Soc. 2015, 12, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, K.; Hyun, M.K.; Jang, E.J.; Lee, N.R.; Yim, J.J. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 2013, 68, 1105–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2021 Report. Available online: https://goldcopd.org/2021-gold-reports/ (accessed on 14 November 2022).
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Agnew, M. Spirometry in clinical use: Practical issues. Breathe 2010, 6, 197–203. [Google Scholar]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 13, 2101499. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.M.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Viegi, G.; Pistelli, F.; Sherrill, D.L.; Maio, S.; Baldacci, S.; Carrozzi, L. Definition, epidemiology and natural history of COPD. Eur. Respir. J. 2007, 30, 993–1013. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, A.; Eriksson, B.; Larsson, L.G.; Rönmark, E.; Sandström, T.; Lundbäck, B. Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest 2006, 129, 879–885. [Google Scholar] [CrossRef]
- Buist, S.; McBurnie, M.A.; Vollmer, W.; Gillespie, S.; Burney, P.; Mannino, D.; Menezes, A.M.B.; Sullivan, S.; Lee, T.; Weiss, K.; et al. International variation in the prevalence of COPD (the BOLD Study): A population-based prevalence study. Lancet 2007, 370, 741–750. [Google Scholar] [CrossRef]
- Tan, W.C.; Sin, D.D.; Bourbeau, J.; Hernandez, P.; Chapman, K.R.; Cowie, R.; FitzGerald, J.M.; Marciniuk, D.D.; Maltais, F.; Buist, A.S.; et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: Results from the CanCOLD study. Thorax 2015, 70, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.K.; Chau, N.Q.; Yunus, F.; Matsunaga, K.; Perng, D.W. COPD Assembly of the APSR. On behalf the COPD Assembly of the APSR. Management of COPD in Asia: A position statement of the Asian Pacific Society of Respirology. Respirology 2019, 24, 1018–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, N.C. Challenges in the management of asthma associated with smoking-induced airway diseases. Expert Opin. Pharmacother. 2018, 19, 1565–1579. [Google Scholar] [CrossRef] [PubMed]
- Güder, G.; Brenner, S.; E Angermann, C.; Ertl, G.; Held, M.; Sachs, A.P.; Lammers, J.-W.; Zanen, P.; Hoes, A.W.; Störk, S.; et al. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir. Res. 2012, 13, 13. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Lee, D.H. How does the pattern of aeroallergen sensitization change over time across all ages? Int. Forum Allergy Rhinol. 2017, 7, 652–659. [Google Scholar] [CrossRef]
- Pallasaho, P.; Rönmark, E.; Haahtela, T.; Sovijärvi, A.A.; Lundbäck, B. Degree and clinical relevance of sensitization to common allergens among adults: A population study in Helsinki, Finland. Clin. Exp. Allergy 2006, 36, 503–509. [Google Scholar] [CrossRef]
- Warm, K.; Backman, H.; Lindberg, A.; Lundbäck, B.; Rönmark, E. Low incidence and high remission of allergic sensitization among adults. J. Allergy Clin. Immunol. 2012, 129, 136–142. [Google Scholar] [CrossRef]
- Scichilone, N.; Callari, A.; Augugliaro, G.; Marchese, M.; Togias, A.; Bellia, V. The impact of age on prevalence of positive skin prick tests and specific IgE tests. Respir. Med. 2011, 105, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Amaral, A.F.; Newson, R.B.; Abramson, M.J.; Antó, J.M.; Bono, R.; Corsico, A.G.; de Marco, R.; Demoly, P.; Forsberg, B.; Gislason, T.; et al. Changes in IgE sensitization and total IgE levels over 20 years of follow-up. J. Allergy Clin. Immunol. 2016, 137, 1788–1795.e9. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, K.; Hildreth, A.J.; Taylor, I.K.; Keaney, N.P.; Bansal, S.K. Predictors of asthma severity in the elderly: Results of a community survey in Northeast England. J. Asthma 1999, 36, 613–618. [Google Scholar] [CrossRef]
- Kaur, R.; Chupp, G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J. Allergy Clin. Immunol. 2019, 144, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kizkin, O.; Turker, G.; Hacievliyagil, S.S.; Gunen, H. Asthma, age, and early reversibility testing. J. Asthma 2003, 40, 317–321. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.; Pesce, G.; Marcon, A.; Accordini, S.; Antonicelli, L.; Bugiani, M.; Casali, L.; Ferrari, M.; Nicolini, G.; Panico, M.G.; et al. The coexistence of asthma and chronic obstructive pulmonary disease (COPD): Prevalence and risk factors in young, middle-aged and elderly people from the general population. PLoS ONE 2013, 8, e62985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israel, E.; Reddel, H.K. Severe and difficult-to-treat asthma in adults. N. Engl. J. Med. 2017, 377, 965–976. [Google Scholar] [CrossRef]
- Sin, D.D.; Miravitlles, M.; Mannino, D.M.; Soriano, J.B.; Price, D.; Celli, B.R.; Leung, J.M.; Nakano, Y.; Park, H.Y.; Wark, P.A.; et al. What is asthma−COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur. Respir. J. 2016, 48, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Orie, N.G.M.; Sluiter, H.J. (Eds.) Bronchitis: An International Symposium; Charles C Thomas: Springfield, IL, USA, 1961. [Google Scholar]
- GINA Report 2017: Asthma, COPD and Asthma-COPD Overlap. Diagnosis and Initial Treatment of Asthma, COPD and Asthma-COPD Overlap. A Joint Project of GINA and GOLD Updated April 2017. Available online: https://ginasthma.org/wp-content/uploads/2019/11/GINA-GOLD-2017-overlap-pocket-guide-wms-2017-ACO.pdf (accessed on 14 November 2022).
- Cosio, B.G.; Soriano, J.B.; López-Campos, J.L.; Calle-Rubio, M.; Soler-Cataluna, J.J.; De-Torres, J.P.; Marín, J.M.; Martínez-Gonzalez, C.; de Lucas, P.; Mir, I.; et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest 2016, 149, 45–52. [Google Scholar] [CrossRef]
- Morgan, B.W.; Grigsby, M.R.; Siddharthan, T.; Chowdhury, M.; Rubinstein, A.; Gutierrez, L.; Irazola, V.; Miranda, J.J.; Bernabe-Ortiz, A.; Alam, D.; et al. Epidemiology and risk factors of asthma-chronic obstructive pulmonary disease overlap in low- and middle-income countries. J. Allergy Clin. Immunol. 2019, 143, 1598–1606. [Google Scholar] [CrossRef]
- Olloquequi, J.; Jaime, S.; Parra, V.; Cornejo-Córdova, E.; Valdivia, G.; Agustí, À.; Silva, O. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir. Res. 2018, 19, 13. [Google Scholar] [CrossRef]



| n (%) | Asthma 146 (26.0) | ACO 181 (32.0) | COPD 241 (42.0) | p | |
|---|---|---|---|---|---|
| |||||
| Sex | NS *; <0.001 †,‡ | ||||
| Female | 106 (18.7) | 50 (34.2) | 54 (29.8) | 2 (0.8) | |
| Male | 462 (81.3) | 96 (65.8) | 127 (70.2) | 239 (99.2) | |
| Age, years | <0.001 *,†,‡ | ||||
| 20–43 | 118 (20.8) | 67 (45.9) | 42 (23.2) | 9 (3.8) | |
| 44–52 | 114 (20.0) | 30 (20.6) | 45 (24.9) | 39 (16.2) | |
| 53–59 | 119 (21.0) | 20 (13.7) | 40 (22.1) | 59 (24.9) | |
| 60–65 | 109 (19.2) | 21 (14.4) | 26 (14.4) | 62 (25.7) | |
| 66–90 | 108 (19.0) | 8 (5.5) | 28 (15.5) | 72 (29.9) | |
| Age, Mean (±95% CI) | 54 (±1) | 46 (±2) | 52 (±2) | 61 (±2) | <0.001 *,†,‡ |
| Level of education | <0.025 *; 0.053 †; <0.01 ‡ | ||||
| Primary | 154 (27.1) | 24 (16.4) | 49 (27.0) | 81 (33.6) | |
| Secondary | 162 (28.5) | 40 (27.4) | 54 (28.8) | 68 (28.2) | |
| High school | 145 (25.5) | 42 (28.8) | 41 (22.7) | 62 (25.8) | |
| Post-secondary | 107 (18.8) | 40 (27.4) | 37 (20.4) | 30 (12.5) | |
| Rural residence | 258 (45.4) | 60 (41.1) | 86 (47.5) | 112 (46.5) | NS *,†,‡ |
| |||||
| History of tuberculosis (+) | 134 (23.6) | 9 (6.2) | 45 (24.9) | 80 (33.2) | <0.001 *,‡; NS † |
| Smoking status | <0.04 *,†,‡ | ||||
| Never | 147 (25.9) | 74 (50.7) | 73 (40.3) | 0 (0) | |
| Ex-smoker | 245 (43.1) | 39 (26.7) | 63 (34.8) | 143 (59.3) | |
| Current smoker | 176 (40.0) | 33 (22.6) | 45 (24.9) | 98 (40.7) | |
| Pack-year (mean ± 95% CI) | 31 (±2) | 16 (±4) | 22 (±4) | 39 (±3) | <0.05 *; <0.001 †,‡ |
| Smoke ≥10Pack-year (+) | 352 (62.0) | 36 (24.7) | 75 (41.4) | 241 (100.0) | <0.01 *; <0.001 †,‡ |
| SPT to mite (+) a | 159 (28.0) | 59 (40.4) | 59 (32.6) | 41 (17.0) | NS *; <0.001 †,‡ |
| IgE to mite (≥0.70 kUA/L) b | 290 (51.1) | 93 (63.7) | 86 (47.5) | 98 (40.7) | NS †; <0.01 *,‡ |
| |||||
| Post-BD FEV1 (%) | 65 (±2) | 82 (±3) | 64 (±3) | 55 (±2) | <0.001 *,†,‡ |
| DLCO (%) | 80 (±2) | 95 (±2) | 83 (±3) | 68 (±3) | <0.001 *,†,‡ |
| All 568 | Asthma 251 (44%) | COPD 317 (56%) | p | |
|---|---|---|---|---|
| Male | 462 (81.3) | 168 (66.9) | 294 (92.7) | <0.0001 |
| Age, years | <0.001 | |||
| 20–43 | 118 (20.8) | 101 (40.2) | 17 (5.4) | |
| 44–52 | 114 (20.0) | 59 (23.5) | 55 (17.4) | |
| 53–59 | 119 (21.0) | 39 (15.5) | 80 (25.2) | |
| 60–65 | 109 (19.2) | 33 (13.1) | 76 (24.0) | |
| 66–90 | 108 (19.0) | 19 (7.6) | 89 (28.1) | |
| Age, Mean (±95% CI) | 54.5 (±1.1) | 47.4 (±1.6) | 60.1 (±1.1) | <0.0001 |
| Level of education | <0.01 | |||
| Primary | 154 (27.1) | 52 (20.7) | 102 (32.2) | |
| Secondary | 162 (28.5) | 65 (25.9) | 97 (30.6) | |
| High school | 145 (25.5) | 72 (28.7) | 73 (23.0) | |
| Post-secondary | 107 (18.8) | 62 (24.7) | 45 (14.2) | |
| House | ||||
| Rural | 258(45.4) | 109 (43.4) | 149 (47.0) | NS |
| Indoor fumes | 476 (83.8) | 191 (76.1) | 285 (89.9) | <0.001 |
| Past tuberculosis | 134 (23.6) | 22 (8.7) | 112 (35.3) | <0.0001 |
| Smoking status | <0.0001 | |||
| Never | 147 (25.9) | 123 (49.0) | 24 (7.6) | |
| Ex-smoker | 245 (43.1) | 74 (29.5) | 171 (53.9) | |
| Current smoker | 176 (40.0) | 54 (21.5) | 122 (38.5) | |
| Smoke ≥10 Pack-year | 352 (62.0) | 71 (28.3) | 281 (88.6) | <0.0001 |
| Pack-year (mean ± 95%CI) | 23.8 (±2.2) | 8.9 (±1.9) | 35.8 (±2.9) | <0.0001 |
| Allergy | ||||
| SPT to mite (+) a | 159 (28.0) | 98 (39.0) | 61 (19.2) | <0.0001 |
| IgE to mite (≥0.70 kUA/L) b | 276 (48.6) | 148 (60.0) | 128 (40.4) | <0.0001 |
| Parameters of lung function [Mean (±95% CI)] | ||||
| Post-BD FEV1 (%) | 65.1 (±1.7) | 77.2 (±2.2) | 55.4 (±2.0) | <0.001 |
| DLCO (%) | 79.8 (±1.9) | 95.4 (±1.7) | 67.5 (±2.2) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.T.; Nguyen, T.C.; Godin, I.; Michel, O. A Proposal to Differentiate ACO, Asthma and COPD in Vietnam. J. Pers. Med. 2023, 13, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13010078
Chu HT, Nguyen TC, Godin I, Michel O. A Proposal to Differentiate ACO, Asthma and COPD in Vietnam. Journal of Personalized Medicine. 2023; 13(1):78. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13010078
Chicago/Turabian StyleChu, Ha Thi, Thuy Chau Nguyen, Isabelle Godin, and Olivier Michel. 2023. "A Proposal to Differentiate ACO, Asthma and COPD in Vietnam" Journal of Personalized Medicine 13, no. 1: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13010078