Prognostic Value of Soluble AXL in Serum from Heart Failure Patients with Preserved and Reduced Left Ventricular Ejection Fraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population and Ethics
2.2. Definition of Study Cohorts and Selection Criteria
2.3. Clinical Assessment at the Time of Inclusion
2.4. Blood Sample Management
2.5. Clinical Laboratory Determinations
2.6. Follow-Up and Major Heart Failure Events Ascertainment
2.7. Statistical Methods
3. Results
3.1. Characteristics of HF cohorts
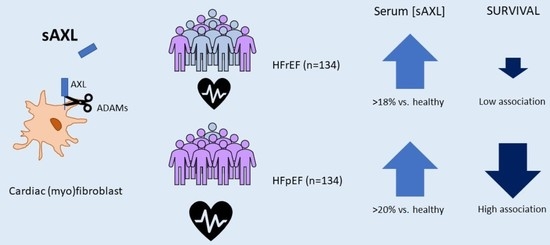
3.2. sAXL Values Are Higher than a Group of Healthy Individuals and Similar in Both HF Groups
3.3. Patient’s Prognosis According to sAXL and NTproBNP Levels in Serum
3.4. Patient’s Characteristics and Prognosis According to sAXL Levels in Serum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Farré, N.; Vela, E.; Clèries, M.; Bustins, M.; Cainzos-Achirica, M.; Enjuanes, C.; Moliner, P.; Ruiz, S.; Verdú-Rotellar, J.M.; Comín-Colet, J. Real world heart failure epidemiology and outcome: A population-based analysis of 88,195 patients. PLoS ONE 2017, 12, e0172745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.; Kozhuharov, N.; Coats, A.J.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [Green Version]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Medicine 2023, 43, 225–236. [Google Scholar] [CrossRef]
- Gui, X.Y.; Rabkin, S.W. C-Reactive Protein, Interleukin-6, Trimethylamine-N-Oxide, Syndecan-1, Nitric Oxide, and Tumor Necrosis Factor Receptor-1 in Heart Failure with Preserved Versus Reduced Ejection Fraction: A Meta-Analysis. Curr. Heart Fail. Rep. 2022. [Google Scholar] [CrossRef]
- Eltelbany, M.; Shah, P.; deFilippi, C. Biomarkers in HFpEF for Diagnosis, Prognosis, and Biological Phenotyping. Curr. Heart Fail. Rep. 2022, 19, 412–424. [Google Scholar] [CrossRef]
- Vedin, O.; Lam, C.S.; Koh, A.S.; Benson, L.; Teng, T.H.K.; Tay, W.T.; Braun, O.Ö.; Savarese, G.; Dahlström, U.; Lund, L.H. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and Reduced Ejection Fraction: A Nationwide Cohort Study. Circ. Heart Fail. 2017, 10, e003875. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Lam, C.S.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868c. [Google Scholar] [CrossRef]
- Stewart, S.; Playford, D.; Scalia, G.M.; Currie, P.; Celermajer, D.S.; Prior, D.; Codde, J.; Strange, G.; NEDA Investigators. Ejection fraction and mortality: A nationwide register-based cohort study of 499 153 women and men. Eur. J. Heart Fail. 2021, 23, 406–416. [Google Scholar] [CrossRef]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef]
- Roberts, E.; Ludman, A.J.; Dworzynski, K.; Al-Mohammad, A.; Cowie, M.R.; McMurray, J.J.; Mant, J. The diagnostic accuracy of the natriuretic peptides in heart failure: Systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015, 350, h910. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Batlle, M.; Recarte-Pelz, P.; Roig, E.; Castel, M.A.; Cardona, M.; Farrero, M.; Ortiz, J.T.; Campos, B.; Pulgarín, M.J.; Ramírez, J.; et al. AXL receptor tyrosine kinase is increased in patients with heart failure. Int. J. Cardiol. 2014, 173, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Ranta, A.; Kumar, S. Recent advancements in role of TAM receptors on efferocytosis, viral infection, autoimmunity, and tissue repair. Int. Rev. Cell Mol. Biol. 2020, 357, 1–19. [Google Scholar] [CrossRef]
- Miller, M.A.; Sullivan, R.J.; Lauffenburger, D.A. Molecular pathways: Receptor ectodomain shedding in treatment, resistance, and monitoring of cancer. Clin. Cancer Res. 2017, 23, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Batlle, M.; Campos, B.; Farrero, M.; Cardona, M.; González, B.; Castel, M.A.; Ortiz, J.; Roig, E.; Pulgarín, M.J.; Ramírez, J.; et al. Use of serum levels of high sensitivity troponin T, galectin-3 and C-terminal propeptide of type I procollagen at long term follow-up in heart failure patients with reduced ejection fraction: Comparison with soluble AXL and BNP. Int. J. Cardiol. 2016, 225, 113–119. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Pan, X.; Ma, T.; Xu, Y.; Fen, Q.; Nijiati, M.; Chi, C.; Su, Y.; Zhang, X.; et al. Prognostic value of plasma sAXL in patients with heart failure: Insights from the DRAGON-HF trial. ESC Heart Fail. 2022, 10, 661–672. [Google Scholar] [CrossRef]
- Caldentey, G.; García De Frutos, P.; Cristóbal, H.; Garabito, M.; Berruezo, A.; Bosch, X.; San Antonio, R.; Flores-Umanzor, E.; Perea, R.J.; De Caralt, T.M.; et al. Serum levels of Growth Arrest-Specific 6 protein and soluble AXL in patients with ST-segment elevation myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 708–716. [Google Scholar] [CrossRef]
- McShane, L.; Tabas, I.; Lemke, G.; Kurowska-Stolarska, M.; Maffia, P. TAM receptors in cardiovascular disease. Cardiovasc. Res. 2019, 115, 1286–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, A.; Aizawa, T.; Mohan, A.; Korshunov, V.A.; Berk, B.C. Hydrogen peroxide activates the Gas6-Axl pathway in vascular smooth muscle cells. J. Biol. Chem. 2004, 279, 28766–28770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Yang, Y.; Wu, X.; Lu, C.; Zhao, H.; Chen, K.; Zhao, A.; Li, X.; Xu, J. GAS6/Axl is associated with AMPK activation and attenuates H2O2-induced oxidative stress. Apoptosis 2022. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, V.A.; Berk, B.C. Flow-Induced Vascular Remodeling in the Mouse: A Model for Carotid Intima-Media Thickening. Arter. Thromb Vasc. Biol. 2003, 23, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Melaragno, M.G.; Wuthrich, D.A.; Poppa, V.; Gill, D.; Lindner, V.; Berk, B.C.; Corson, M.A. Increased expression of Axl tyrosine kinase after vascular injury and regulation by G protein-coupled receptor agonists in rats. Circ. Res. 1998, 83, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Van Beusecum, J.P.; Xiao, L.; Patrick, D.M.; Ao, M.; Zhao, S.; Lopez, M.G.; Billings, F.T., 4th; Cavinato, C.; Caulk, A.W.; et al. Role of Axl in target organ inflammation and damage due to hypertensive aortic remodeling. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H917–H933. [Google Scholar] [CrossRef]
- Batlle, M.; Castillo, N.; Alcarraz, A.; Sarvari, S.; Sangüesa, G.; Cristóbal, H.; García de Frutos, P.; Sitges, M.; Mont, L.; Guasch, E. Axl expression is increased in early stages of left ventricular remodeling in an animal model with pressure-overload. PLoS ONE 2019, 14, e0217926. [Google Scholar] [CrossRef]
- DeBerge, M.; Glinton, K.; Subramanian, M.; Wilsbacher, L.D.; Rothlin, C.V.; Tabas, I.; Thorp, E.B. Macrophage AXL receptor tyrosine kinase inflames the heart after reperfused myocardial infarction. J. Clin. Investig. 2021, 131, e139576. [Google Scholar] [CrossRef]
- Tutusaus, A.; de Gregorio, E.; Cucarull, B.; Cristóbal, H.; Aresté, C.; Graupera, I.; Coll, M.; Colell, A.; Gausdal, G.; Lorens, J.B.; et al. A Functional Role of GAS6/TAM in Nonalcoholic Steatohepatitis Progression Implicates AXL as Therapeutic Target. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 349–368. [Google Scholar] [CrossRef]
- Glinton, K.; DeBerge, M.; Fisher, E.; Schroth, S.; Sinha, A.; Wang, J.J.; Wasserstrom, J.A.; Ansari, M.J.; Zhang, Z.J.; Feinstein, M.; et al. Bone marrow-derived AXL tyrosine kinase promotes mitogenic crosstalk and cardiac allograft vasculopathy. J. Heart Lung Transpl. 2021, 40, 435–446. [Google Scholar] [CrossRef]
- Tjwa, M.; Bellido-Martin, L.; Lin, Y.; Lutgens, E.; Plaisance, S.; Bono, F.; Delesque-Touchard, N.; Hervé, C.; Moura, R.; Billiau, A.D.; et al. Gas6 promotes inflammation by enhancing interactions between endothelial cells, platelets, and leukocytes. Blood 2008, 111, 4096–4105. [Google Scholar] [CrossRef] [Green Version]
- Gavaldà-Manso, M.; Jimenez-Marrero, S.; Cainzos-Achirica, M.; Garay, A.; Enjuanes, C.; Yun, S.; Diez, C.; Gonzalez-Costello, J.; Tajes, M.; Farre, N.; et al. Reduced levels of vasopressin, an independent mechanism in the obesity paradox in patients with chronic heart failure: Insights from the DAMOCLES study. Int. J. Cardiol. 2019, 276, 171–176. [Google Scholar] [CrossRef]
- Díez-López, C.; Tajes Orduña, M.; Enjuanes Grau, C.; Moliner Borja, P.; González-Costello, J.; García-Romero, E.; Francesch Manzano, J.; Yun Viladomat, S.; Jiménez-Marrero, S.; Ramos-Polo, R.; et al. Blood Differential Gene Expression in Patients with Chronic Heart Failure and Systemic Iron Deficiency: Pathways Involved in Pathophysiology and Impact on Clinical Outcomes. J. Clin. Med. 2021, 10, 4937. [Google Scholar] [CrossRef]
- Martínez-Bosch, N.; Cristóbal, H.; Iglesias, M.; Gironella, M.; Barranco, L.; Visa, L.; Calafato, D.; Jiménez-Parrado, S.; Earl, J.; Carrato, A.; et al. Soluble AXL is a novel blood marker for early detection of pancreatic ductal adenocarcinoma and differential diagnosis from chronic pancreatitis. EBioMedicine 2022, 75, 103797. [Google Scholar] [CrossRef]
- Dengler, M.; Huber, H.; Müller, C.J.; Zellmer, A.; Rauch, P.; Mikulits, W. Accurate Determination of Soluble Axl by Enzyme-Linked Immunosorbent Assay. Assay Drug Dev. Technol. 2016, 14, 543–550. [Google Scholar] [CrossRef]
- Gama, R.M.; Peracha, J.; Bramham, K.; Cockwell, P. Removal of ethnicity adjustment for creatinine-based estimated glomerular filtration rate equations. Ann Clin Biochem. 2023, 00045632221149660. [Google Scholar] [CrossRef]
- Mirabet, S.; García-Osuna, A.; Garcia de Frutos, P.; Ferrero-Gregori, A.; Brossa, V.; Lopez, L.; Leta, R.; Garcia-Picart, J.; Padro, J.M.; Sánchez-Quesada, J.L.; et al. High-Sensitivity Troponin T and Soluble Form of AXL as Long-Term Prognostic Biomarkers after Heart Transplantation. Dis Markers 2018, 2018, 6243529. [Google Scholar] [CrossRef]
- Santema, B.T.; Kloosterman, M.; Van Gelder, I.C.; Mordi, I.; Lang, C.C.; Lam, C.S.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; et al. Comparing biomarker profiles of patients with heart failure: Atrial fibrillation vs. sinus rhythm and reduced vs. preserved ejection fraction. Eur. Heart J. 2018, 39, 3867–3875. [Google Scholar] [CrossRef] [Green Version]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.; Kok, W.E. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.S.P.; Gamble, G.D.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Cameron, V.A.; et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur. Heart J. 2018, 39, 1770–1780. [Google Scholar] [CrossRef] [Green Version]
- Torrente-Rodríguez, R.M.; Martín, C.M.; Gamella, M.; Pedrero, M.; Martínez-Bosch, N.; Navarro, P.; García de Frutos, P.; Pingarrón, J.M.; Campuzano, S. Electrochemical Immunosensing of ST2: A Checkpoint Target in Cancer Diseases. Biosensors 2021, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupón, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT-proBNP and High-Sensitivity troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Ponikowska, B.; Iwanek, G.; Zdanowicz, A.; Urban, S.; Zymliński, R.; Ponikowski, P.; Biegus, J. Biomarkers of Myocardial Injury and Remodeling in Heart Failure. J. Pers. Med. 2022, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Agdashian, D.; Daniels, L.B. What Is the Clinical Utility of Cardiac Troponins in Heart Failure? Are They Modifiable Beyond Their Prognostic Value? Curr. Heart Fail. Rep. 2023. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L., Jr.; Vergaro, G.; Ripoli, A.; Latini, R.; Masson, S.; Magnoli, M.; Anand, I.S.; Cohn, J.N.; Tavazzi, L.; et al. Prognostic Value of High-Sensitivity Troponin T in Chronic Heart Failure: An Individual Patient Data Meta-Analysis. Circulation 2018, 137, 286–297. [Google Scholar] [CrossRef]
- Tutusaus, A.; Marí, M.; Ortiz-Pérez, J.T.; Nicolaes, G.A.F.; Morales, A.; García de Frutos, P. Role of Vitamin K-Dependent Factors Protein S and GAS6 and TAM Receptors in SARS-CoV-2 Infection and COVID-19-Associated Immunothrombosis. Cells 2020, 9, 2186. [Google Scholar] [CrossRef]
- Bárcena, C.; Stefanovic, M.; Tutusaus, A.; Joannas, L.; Menéndez, A.; García-Ruiz, C.; Sancho-Bru, P.; Marí, M.; Caballeria, J.; Rothlin, C.V.; et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J. Hepatol. 2015, 63, 670–678. [Google Scholar] [CrossRef] [Green Version]


| HFrEF | HFpEF | p | |
|---|---|---|---|
| Number | 134 | 134 | |
| Demographics | |||
| Age (years) | 71 (61–78) | 78 (71–82) | <0.001 |
| Female, n (%) | 41 (30.6) | 87 (64.9) | <0.001 |
| Risk Factors, n (%) | |||
| Diabetes, n (%) | 63 (47.0) | 73 (54.5) | 0.222 |
| Ischemic etiology, n (%) | 68 (50.7) | 26 (19.4) | <0.001 |
| Clinical characteristics and outcomes | |||
| BMI (kg/m2) | 27.3 (24.4–30.8) | 29.5 (26.3–33.3) | 0.001 |
| NYHA FC I-II / III-IV, n (%) | 89 (66.4)/45 (33.6) | 70 (52.2)/64 (47.8) | 0.018 |
| EF | 32 (31–33) | 62 (60–64) | <0.001 |
| SBP (mmHg) | 120 (107–130) | 129 (115–141) | <0.001 |
| Comorbodities number > 4, n (%) | 59 (44.4) | 69 (53.4) | 0.088 |
| All-cause mortality | 64 (47.8) | 53 (39.6) | 0.175 |
| Cardiovascular mortality | 20 (14.9) | 20 (14.9) | 1.000 |
| Re-admission | 49 (36.6) | 42 (31.3) | 0.367 |
| Re-admission or exitus (all-cause) | 80 (59.7) | 75 (56.0) | 0.536 |
| Re-admission or exitus (CV) | 54 (40.3) | 51 (38.1) | 0.707 |
| Laboratory variables | |||
| eGFR | 60.6 (47.0–76.3) | 52.5 (38.0–68.7) | 0.009 |
| sAXL (ng/mL) | 37.2 (28.4–46.8) | 37.9 (30.0–44.5) | 0.612 |
| NT-proBNP | 3.22 (2.78–3.54) | 3.06 (2.78–3.43) | 0.061 |
| Medications (%) | |||
| ACE/ARB, n (%) | 111 (82.8) | 90 (67.2) | 0.003 |
| β-blocker, n (%) | 128 (95.5) | 109 (81.3) | <0.001 |
| Hydralazine, n (%) | 23 (17.2) | 31 (23.1) | 0.235 |
| HFrEF | HFpEF | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| All-cause mortality | ||||
| sAXL | 1.019 (1.000–1.038) | 0.047 | 1.032 (1.013–1.052) | <0.001 |
| NT-proBNP | 1.050 (1.036–1.066) | <0.001 | 1.021 (1.021–1.050) | <0.001 |
| Cardiovascular mortality | ||||
| sAXL | 0.991 (0.954–1.030) | 0.647 | 1.054 (1.025–1.083) | <0.001 |
| NT-proBNP | 1.065 (1.040–1.091) | <0.001 | 1.041 (1.020–1.063) | <0.001 |
| Re-admission | ||||
| sAXL | 1.000 (0.977–1.024) | 0.995 | 1.002 (0.977–1.027) | 0.904 |
| NT-proBNP | 1.037 (1.019–1.055) | <0.001 | 1.025 (1.006–1.044) | 0.011 |
| Re-admission or exitus (all-cause) | ||||
| sAXL | 1.010 (0.993–1.027) | 0.27 | 1.020 (1.003–1.037) | 0.020 |
| NT-proBNP | 1.042 (1.028–1.055) | <0.001 | 1.030 (1.016–1.045) | <0.001 |
| Re-admission or exitus (Cardiovascular) | ||||
| sAXL | 1.002 (0.981–1.024) | 0.832 | 1.022 (1.002–1.042) | 0.028 |
| NT-proBNP | 1.041 (1.024–1.058) | <0.001 | 1.032 (1.015–1.048) | <0.001 |
| HFrEF | HFpEF | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| All-cause mortality | ||||||||
| sAXL | 1.018 (0.998–1.037) | 0.073 | 1.005 (0.982–1.028) | 0.693 | 1.028 (1.008–1.048) | 0.005 | 1.024 (1.002–1.046) | 0.033 |
| Age | 1.069 (1.041–1.098) | <0.001 | 1.042 (1.046–1.076) | 0.002 | 1.024 (0.989–1.061) | 0.186 | 1.030 (0.868–2.959) | 0.132 |
| NYHA class | 1.463 (0.877–2.441) | 0.145 | 1.238 (0.737–2.079) | 0.420 | 1.805 (0.993–3.280) | 0.053 | 1.603 (0.868–2.959) | 0.132 |
| NTproBNP | 1.037 (1.020–1.055) | <0.001 | 1.026 (1.009–1.044) | 0.003 | ||||
| eGFR | 0.999 (0.988–1.010) | 0.788 | 0.989 (0.974–1.005) | 0.182 | ||||
| Cardiovascular mortality | ||||||||
| sAXL | 0.989 (0.951–1.029) | 0.586 | 0.952 (0.907–1.000) | 0.045 | 1.051 (1.022–1.081) | <0.001 | 1.049 (1.018–1.081) | 0.002 |
| Age | 1.066 (1.018–1.115) | 0.006 | 1.023 (0.978–1.070) | 0.264 | 1.054 (0.988–1.124) | 0.103 | 1.077 (1.007–1.153) | 0.030 |
| NYHA class | 1.625 (0.663–3.982) | 0.288 | 1.520 (0.596–3.877) | 0.429 | 1.706 (0.632–1.124) | 0.292 | 1.522 (0.551–4.209) | 0.418 |
| NTproBNP | 11.786 (3.61–38.46) | <0.001 | 1.034 (1.007–1.062) | 0.013 | ||||
| eGFR | 1.004 (0.989–1.019) | 0.472 | 1.003 (0.978–1.028) | 0.833 | ||||
| HFrEF | HFpEF | |||||
|---|---|---|---|---|---|---|
| sAXL ≤ Q3 | sAXL > Q3 | P | sAXL ≤ Q3 | sAXL > Q3 | P | |
| Number | 101 | 33 | 101 | 33 | ||
| AC mortality n (%) | 45 (44.5) | 19 (57.6) | 0.136 | 35 (34.6) | 18 (54.5) | 0.035 |
| CV mortality n (%) | 3 (9.01) | 17 (16.8) | 0.279 | 11 (10.9) | 9 (27.3) | 0.022 |
| NYHA III-IV | 32 (31.7) | 13 (39.4) | 0.271 | 47 (46.5) | 17 (51.5) | 0.383 |
| SBP (mm Hg) | 120 (106–130) | 120 (110-131) | 0.329 | 129 (115-140) | 129 (113-147) | 0.616 |
| Re-ad. or exitus n (%) | 40 (39.6) | 14 (42.4) | 0.465 | 34 (33.7) | 17 (51.5) | 0.053 |
| Sex (male) n (%) | 72 (71.3) | 21 (63.6) | 0.268 | 38 (37.6) | 9 (27.3) | 0.193 |
| Diabetes n (%) | 45 (44.5) | 18 (54.5) | 0.213 | 54 (53.5) | 19 (57.6) | 0.418 |
| eGFR | 62.2 (52.5–78.2) | 42.1 (23.9–66.2) | <0.001 | 52.5 (38.5) | 52.4 (34.8–69.2) | 0.784 |
| BMI | 27.3 (24.1–30.8) | 27.3 (24.9–30.7) | 0.749 | 29.6 (26.3–33.0) | 27.8 (25.4–35.6) | 0.848 |
| Age (years) | 69 (60.0–78.0) | 75 (67.5–78.5) | 0.069 | 78.0 (71.0–83.0) | 77.5 (71.5–79.5) | 0.776 |
| NT-proBNP | 3.13 (2.73–3.49) | 3.49 (3.15–3.96) | <0.001 | 3.02 (2.76–3.33) | 3.28 (2.84–3.63) | 0.050 |
| Hemoglobin (g/dL) | 13.1 (12.3–14.2) | 12.4 (10.8–13.4) | 0.005 | 12.2 (11.05–14) | 11.1 (10.0–11.9) | <0.001 |
| HFrEF | HFpEF | |
|---|---|---|
| Q1 | 1 | 1 |
| Q2 | 0.621 (0.291–1.327) | 2.222 (0.883–5.592) |
| Q3 | 0.947 (0.466–1.923) | 1.908 (0.776–4.690) |
| Q4 | 1.584 (0.837–2.988) | 3.628 (1.499–8.782) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristóbal, H.; Enjuanes, C.; Batlle, M.; Tajes, M.; Campos, B.; Francesch, J.; Moliner, P.; Farrero, M.; Andrea, R.; Ortiz-Pérez, J.T.; et al. Prognostic Value of Soluble AXL in Serum from Heart Failure Patients with Preserved and Reduced Left Ventricular Ejection Fraction. J. Pers. Med. 2023, 13, 446. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13030446
Cristóbal H, Enjuanes C, Batlle M, Tajes M, Campos B, Francesch J, Moliner P, Farrero M, Andrea R, Ortiz-Pérez JT, et al. Prognostic Value of Soluble AXL in Serum from Heart Failure Patients with Preserved and Reduced Left Ventricular Ejection Fraction. Journal of Personalized Medicine. 2023; 13(3):446. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13030446
Chicago/Turabian StyleCristóbal, Helena, Cristina Enjuanes, Montserrat Batlle, Marta Tajes, Begoña Campos, Josep Francesch, Pedro Moliner, Marta Farrero, Rut Andrea, José Tomás Ortiz-Pérez, and et al. 2023. "Prognostic Value of Soluble AXL in Serum from Heart Failure Patients with Preserved and Reduced Left Ventricular Ejection Fraction" Journal of Personalized Medicine 13, no. 3: 446. https://0-doi-org.brum.beds.ac.uk/10.3390/jpm13030446