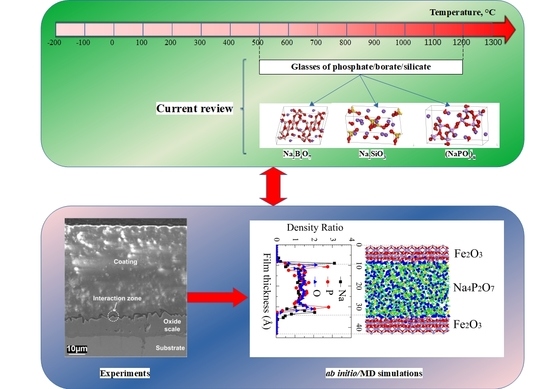
Tribochemistry and Lubrication of Alkaline Glass Lubricants in Hot Steel Manufacturing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Studies
2.1.1. Sodium Polyphosphate
2.1.2. Sodium Borate
2.1.3. Sodium Silicate
2.2. Theoretical Simulations
2.2.1. Phosphate Glass
2.2.2. Borate Glass
2.2.3. Silicate Glass
2.2.4. The Roles of Na in Glass Lubricants
2.2.5. Reactive Molecular Dynamics Lubrication Simulations
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Cui, S.; Wan, S.; Zhu, Q.; Tieu, A.K.; Zhu, H.; Wang, L.; Cowie, B. Tribochemical Behavior of Phosphate Compounds at an Elevated Temperature. J. Phys. Chem. C 2016, 120, 25742–25751. [Google Scholar] [CrossRef]
- Beynon, J.H. Tribology of hot metal forming. Tribol. Int. 1998, 31, 73–77. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yuen, W.Y.D. Oxide-Scale Structures Formed on Commercial Hot-Rolled Steel Strip and Their Formation Mechanisms. Oxid. Met. 2001, 56, 89–118. [Google Scholar] [CrossRef]
- Schey, J.A. Tribology in Metalworking: Friction, Lubrication, and Wear. J. Appl. Met. 1984, 3, 173. [Google Scholar] [CrossRef]
- Shirizly, A.; Lenard, J.G. The effect of lubrication on mill loads during hot rolling of low carbon steel strips. J. Mater. Process. Technol. 2000, 97, 61–68. [Google Scholar] [CrossRef]
- Shirizly, A.; Lenard, J.G. The effect of scaling and emulsion delivery on heat transfer during the hot rolling of steel strips. J. Mater. Process. Technol. 2000, 101, 250–259. [Google Scholar] [CrossRef]
- Azushima, A.; Xue, W.; Yoshida, Y. Influence of Lubricant Factors on Coefficient of Friction and Clarification of Lubrication Mechanism in Hot Rolling. ISIJ Int. 2009, 49, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Allam, I.M. Solid lubricants for applications at elevated temperatures. J. Mater. Sci. 1991, 26, 3977–3984. [Google Scholar] [CrossRef]
- Bay, N.O.; Azushima, A.; Groche, P.; Ishibashi, I.; Merklein, M.; Morishita, M.; Nakamura, T.; Schmid, S.; Yoshida, M. Environmentally benign tribo-systems for metal forming. CIRP Ann. 2010, 59, 760–780. [Google Scholar] [CrossRef] [Green Version]
- Feher, R. Graphite-Based Lubricants. In Encyclopedia of Lubricants and Lubrication; Mang, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 758–769. [Google Scholar] [CrossRef]
- Friedman, P.A.; Luckey, S.G. High-Temperature Lubricants for Superplastic Forming of Metals. In Superplastic Forming of Advanced Metallic Materials; Giuliano, G., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 72–82. [Google Scholar] [CrossRef]
- Voevodin, A.; Muratore, C.; Aouadi, S. Hard coatings with high temperature adaptive lubrication and contact thermal management: Review. Surf. Coat. Technol. 2014, 257, 247–265. [Google Scholar] [CrossRef]
- Erdemir, A. Solid Lubricants and Self Lubricating Films. In Modern Handbook of Tribology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Shi, X.; Zhai, W.; Wang, M.; Xu, Z.; Yao, J.; Song, S.; Wang, Y. Tribological behaviors of NiAl based self-lubricating composites containing different solid lubricants at elevated temperatures. Wear 2014, 310, 1–11. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, Q.; Yang, J.; Liu, W.; Xue, Q. Ni3Al matrix high temperature self-lubricating composites. Tribol. Int. 2011, 44, 445–453. [Google Scholar] [CrossRef]
- Zhu, S.; Cheng, J.; Qiao, Z.; Yang, J. High temperature solid-lubricating materials: A review. Tribol. Int. 2019, 133, 206–223. [Google Scholar] [CrossRef]
- Johnson, R.L.; Swikert, M.A.; Buckley, D.H. High Temperature Lubrication in Reactive Atmospheres. Corrosion 1960, 16, 395t–398t. [Google Scholar] [CrossRef]
- Tieu, A.K.; Kong, N.; Wan, S.; Zhu, H.; Zhu, Q.; Mitchell, D.R.; Kong, C. The Influence of Alkali Metal Polyphosphate on the Tribological Properties of Heavily Loaded Steel on Steel Contacts at Elevated Temperatures. Adv. Mater. Interfaces 2015, 2, 1500032. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Deng, G.; Wang, J.; Tran, B.H.; Zhu, H.; Yang, J. In-situ interfacial tribochemistry toward eliminating red-scale of silicon steel in friction process. Tribol. Int. 2020, 143, 106077. [Google Scholar] [CrossRef]
- Cui, S.; Zhu, H.; Wan, S.; Tran, B.; Wang, L.; Tieu, K.A. Investigation of different inorganic chemical compounds as hot metal forming lubricant by pin-on-disc and hot rolling. Tribol. Int. 2018, 125, 110–120. [Google Scholar] [CrossRef]
- Wan, S.; Tieu, A.K.; Zhu, Q.; Zhu, H.; Cui, S.; Mitchell, D.R.; Kong, C.; Cowie, B.; Denman, J.A.; Liu, R. Chemical nature of alkaline polyphosphate boundary film at heated rubbing surfaces. Sci. Rep. 2016, 6, 26008. [Google Scholar] [CrossRef]
- Tran, B.H.; Tieu, K.; Wan, S.; Zhu, H.; Cui, S.; Wang, L. Understanding the tribological impacts of alkali element on lubrication of binary borate melt. RSC Adv. 2018, 8, 28847–28860. [Google Scholar] [CrossRef] [Green Version]
- Tran, B.H.; Tieu, A.K.; Wan, S.; Zhu, H.; Liu, R. Hot corrosion of borate melt and interface chemistry of borate-coated steel under tribological stimulation. Corros. Sci. 2018, 140, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Tran, B.H.; Wan, S.; Tieu, A.K.; Zhu, H. Tribological performance of inorganic borate at elevated temperatures. Tribol. Trans. 2020, 1–9. [Google Scholar] [CrossRef]
- Ta, H.T.; Tieu, A.K.; Zhu, H.; Yu, H.; Tran, N.V.; Tran, B.H.; Wan, S.; Ta, T.D. Ab initio study on physical and chemical interactions at borates and iron oxide interface at high temperature. Chem. Phys. 2020, 529, 110548. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Zhu, H.; Cui, S.; Deng, G.; Hai, G.; Yang, J. Contribution of Sodium Metasilicate to the Diffusion of Mn in Steel under Tribological Contact at High Temperatures. J. Phys. Chem. C 2019, 123, 14468–14479. [Google Scholar] [CrossRef]
- Matsumoto, K.; Izawa, M.; Nakanishi, T.; Tsubouchi, K. Tribological Properties of Water Glass Lubricant for Hot Metalworking. Tribol. Trans. 2009, 52, 553–559. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Cui, S.; Deng, G.; Wang, P.; Zhu, H.; Yang, J.; Wamg, P. Lubrication mechanism of sodium metasilicate at elevated temperatures through tribo-interface observation. Tribol. Int. 2020, 142, 105972. [Google Scholar] [CrossRef]
- Wang, L.; Tieu, A.K.; Zhu, H.; Deng, G.; Hai, G.; Wang, J.; Yang, J. The effect of expanded graphite with sodium metasilicate as lubricant at high temperature. Carbon 2020, 159, 345–356. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.; Çaǧın, T.; Yamaguchi, E.S.; Frazier, R.; Ho, A.; Tang, Y.; Goddard, W.A.; Çağın, T. Application of the Self-Assembled Monolayer (SAM) Model to Dithiophosphate and Dithiocarbamate Engine Wear Inhibitors. J. Phys. Chem. A 2000, 104, 2508–2524. [Google Scholar] [CrossRef]
- Ta, H.T.T.; Tieu, A.K.; Zhu, H.; Yu, H.; Ta, T.D.; Wan, S.; Tran, N.V.; Le, H.M. Chemical Origin of Sodium Phosphate Interactions on Iron and Iron Oxide Surfaces by First Principle Calculations. J. Phys. Chem. C 2017, 122, 635–647. [Google Scholar] [CrossRef]
- Le, H.M.; Tieu, A.K.; Zhu, H.; Ta, T.D.; Yu, H.; Ta, T.T.H.; Tran, V.N.; Wan, S. Depolymerization of sodium polyphosphates on an iron oxide surface at high temperature. Phys. Chem. Chem. Phys. 2018, 20, 7819–7835. [Google Scholar] [CrossRef] [Green Version]
- Le, M.H.; Tieu, A.K.; Zhu, H.; Ta, D.T.; Yu, H.; Ta, T.T.H.; Tran, V.N. Surface Transformation and Interactions of Iron Oxide in Glassy Lubricant: An Ab Initio Study. Chem. Phys. 2020. proofreading. [Google Scholar]
- Ta, T.D.; Le, H.M.; Tieu, A.K.; Zhu, H.; Ta, H.T.T.; Tran, N.V.; Wan, S.; Xiao, J. Reactive Molecular Dynamics Study of Hierarchical Tribochemical Lubricant Films at Elevated Temperatures. ACS Appl. Nano Mater. 2020, 3, 2687–2704. [Google Scholar] [CrossRef]
- Ta, H.T.T.; Tieu, A.K.; Zhu, H.; Yu, H.; Tran, N.V.; Ta, T.D. Mechanisms of Pressure-Induced Structural Transformation in Confined Sodium Borate Glasses. J. Phys. Chem. B 2019, 124, 277–287. [Google Scholar] [CrossRef]
- Ta, H.T.T.; Tieu, K.A.; Zhu, H.; Yu, H.; Tran, N.V.; Le, H.M.; Ta, T.D. Structural Response of Alkali Metal Borates at Sliding Interface: The Effect of Alkali Cations. Comput. Mater. Sci. 2020. under review. [Google Scholar]
- Tran, N.V.; Tieu, A.K.; Zhu, H.; Ta, H.T.; Sang, P.T.; Le, H.M.; Ta, T.D. Insights into the tribochemistry of sliding iron oxide surfaces lubricated by sodium silicate glasses: An ab initio molecular dynamics study. Appl. Surf. Sci. 2020, 528, 147008. [Google Scholar] [CrossRef]
- Tran, N.V.; Tieu, A.K.; Zhu, H.; Ta, H.T.T.; Ta, T.D.; Le, H.M. First-Principles Study of the Adsorption and Depolymerization Mechanisms of Sodium Silicate on Iron Surfaces at High Temperature. J. Phys. Chem. C 2018, 122, 20827–20840. [Google Scholar] [CrossRef]
- Riga, A.; Cahoon, J.; Pistillo, W.R. Organophosphorus chemistry structure and performance relationships in FZG gear tests. Tribol. Lett. 2001, 9, 219–225. [Google Scholar] [CrossRef]
- Asensio, M.C.; Righi, M.C.; Philippon, D.; Mambingo-Doumbe, S.; Le-Mogne, T.; Martin, J.M.; Bouffet, A. Tribochemistry of phosphorus additives: Experiments and first-principles calculations. RSC Adv. 2015, 5, 49270–49279. [Google Scholar] [CrossRef] [Green Version]
- Jabraoui, H.; Vaills, Y.; Hasnaoui, A.; Badawi, M.; Ouaskit, S. Effect of Sodium Oxide Modifier on Structural and Elastic Properties of Silicate Glass. J. Phys. Chem. B 2016, 120, 13193–13205. [Google Scholar] [CrossRef]
- Tran, N.V.; Tieu, A.K.; Zhu, H.; Ta, H.T.T.; Le, H.M.; Ta, T.D. An ab initio study on the effects of Na passivation on friction reduction of an iron oxide surface. J. Appl. Phys. 2020, 127, 065305. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ta, T.D.; Tran, B.H.; Tieu, K. Tribochemistry and Lubrication of Alkaline Glass Lubricants in Hot Steel Manufacturing. Lubricants 2020, 8, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/lubricants8070070
Ta TD, Tran BH, Tieu K. Tribochemistry and Lubrication of Alkaline Glass Lubricants in Hot Steel Manufacturing. Lubricants. 2020; 8(7):70. https://0-doi-org.brum.beds.ac.uk/10.3390/lubricants8070070
Chicago/Turabian StyleTa, Thi D., Bach H. Tran, and Kiet Tieu. 2020. "Tribochemistry and Lubrication of Alkaline Glass Lubricants in Hot Steel Manufacturing" Lubricants 8, no. 7: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/lubricants8070070