Wolbachia’s Deleterious Impact on Aedes aegypti Egg Development: The Potential Role of Nutritional Parasitism
Abstract
:Simple Summary
Abstract
1. Mosquito Biocontrol Methods
2. Impacts of Wolbachia on Host Fitness
2.1. Wolbachia Impacts on Host Fitness during Aquatic and Adult Life Stages
2.2. Wolbachia Impacts on Egg Survival
3. Wolbachia Genome Indicates Its Dependence on Host Resources
4. Nutrient Quantification Studies
5. Nutritional Requirements for Egg Production
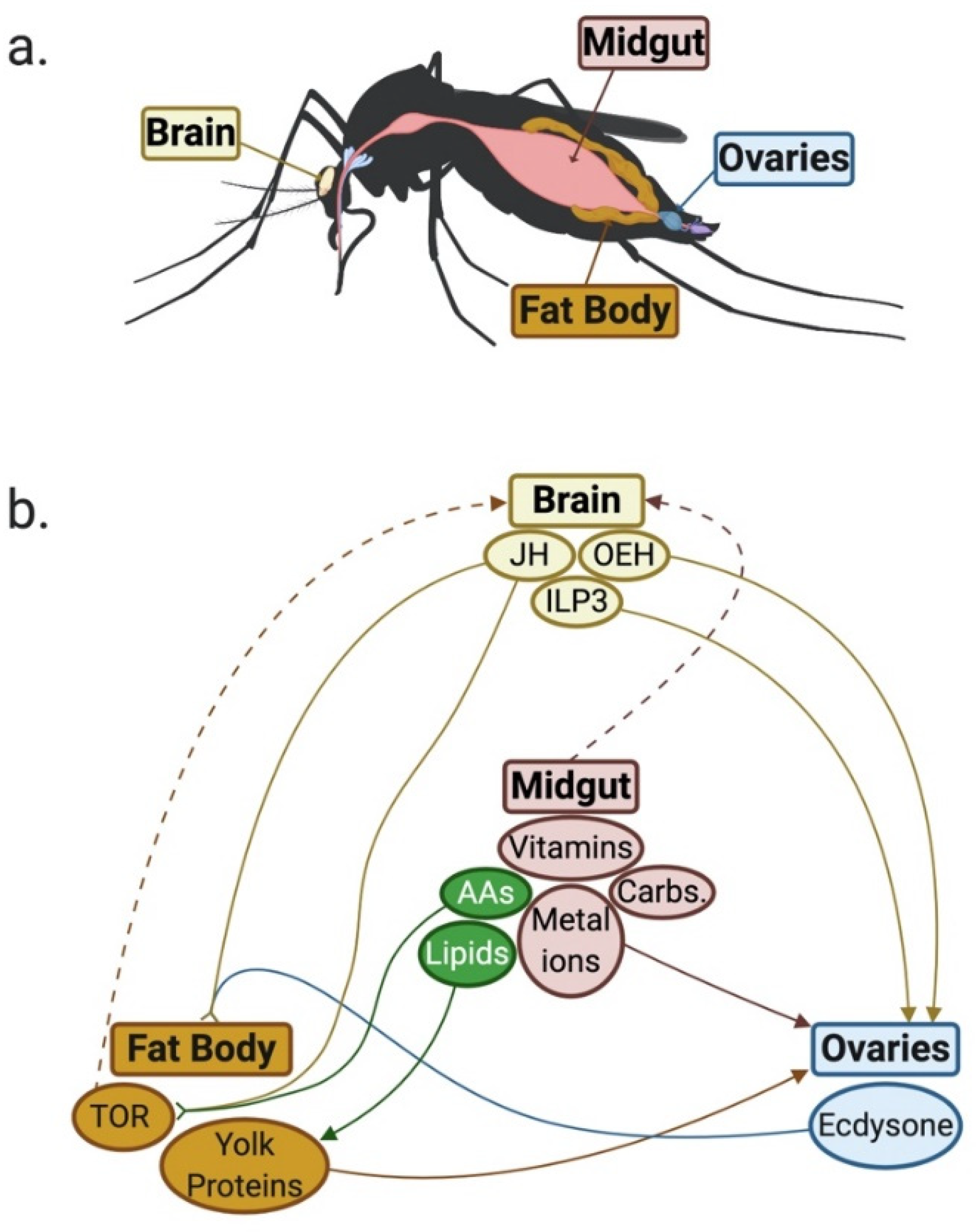
5.1. Process of Egg Production in Aedes aegypti
5.2. Nutritional Requirements for Aedes aegypti and Drosophila Egg Production
5.2.1. Amino Acids
5.2.2. Lipids
5.2.3. Other Nutrients
6. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Oliveira, L.N.D.S.; Itria, A.; Lima, E.C. Cost of illness and program of dengue: A systematic review. PLoS ONE 2019, 14, e0211401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelker, R. Dengue Vaccine Gets the Nod. JAMA 2019, 321, 2066. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Deen, J.; Buchy, P.; Gubler, D.; Harris, E.; Homach, J. World Health Organization Dengue Guidelines for Diagnosis, Treatment, Prevention, and Control; WHO Publisher: Geneva, Switzerland, 2009; p. 3. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Suwanmanee, S.; Luplertlop, N. Dengue and Zika viruses: Lessons learned from the similarities between these Aedes mosquito-vectored arboviruses. J. Microbiol. 2017, 55, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Barzon, L.; Pijlman, G.P.; de la Fuente, J.; Rizzoli, A.; Wammes, L.J.; Takken, W.; van Rij, R.P.; Papa, A. Human to human transmission of arthropod-borne pathogens. Curr. Opin. Virol. 2017, 22, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti vector competence studies: A. review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Pigott, D.M.; Golding, N.; Kraemer, M.U.G.; Scott, T.W.; Wint, G.R.W.; Smith, D.L.; Hay, S.I. The many projected futures of dengue. Nat. Rev. Microbiol. 2015, 13, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Zhang, W.; Guo, L.R. Evolution and phylogeny of Wolbachia: Reproductive parasites of arthropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1995, 261, 55–63. [Google Scholar] [CrossRef]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito—Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef] [PubMed]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Zug, R.; Hammerstein, P. Still a Host of Hosts for Wolbachia: Analysis of Recent Data Suggests That 40% of Terrestrial Arthropod Species Are Infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.C.; Sidhu, M.; Wang, Y.-F.; O’Neill, S.L. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, P.A.; Callahan, A.G.; Yang, Q.; Jasper, M.; Arif, M.A.K.; Afizah, A.N.; Nazni, W.A.; Hoffmann, A.A. An elusive endosymbiont: Does Wolbachia occur naturally in Aedes aegypti? Ecol. Evol. 2020, 10, 1581–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, J.E.; de Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Turelli, M. Unidirectional incompatibility in Drosophila simulans: Inheritance, geographic variation and fitness effects. Genetics 1988, 119, 435–444. [Google Scholar]
- Turelli, M.; Hoffmann, A.A. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 1991, 353, 440–442. [Google Scholar] [CrossRef]
- Turelli, M.; Hoffmann, A.A. Cytoplasmic incompatibility in Drosophila simulans: Dynamics and parameter estimates from natural populations. Genetics 1995, 140, 1319–1338. [Google Scholar]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.H.; Carrasco, A.M.; Dong, Y.; Sgrò, C.M.; McGraw, E.A. The effect of temperature on Wolbachia-mediated dengue virus blocking in Aedes aegypti. Am. J. Trop. Med. Hyg. 2016, 94, 812–819. [Google Scholar] [CrossRef] [Green Version]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Vo, L.T.; et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. USA 2017, 115, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; da Silva, V.L.; Fernandes, M.D.R.; Logullo, C.; Maciel-de-Freitas, R.; Moreira, L.A. The influence of larval competition on Brazilian Wolbachia-infected Aedes aegypti mosquitoes. Parasites Vectors 2016, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Hurk, A.F.V.D.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res. 2019, 3, 1547. [Google Scholar] [CrossRef]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.R.; Arif, M.A.K.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr. Biol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, J.A.; Melton, L. Verily project releases millions of factory-reared mosquitoes. Nat. Biotechnol. 2018, 36, 781. [Google Scholar] [CrossRef]
- O’Neill, S.L. Dengue and Zika: Control and antiviral treatment strategies. Adv. Exp. Med. Biol. 2018, 1062, 355–360. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Addison, M.F.; Terblanche, J.S. Mass-rearing of insects for pest management: Challenges, synergies and advances from evolutionary physiology. Crop Prot. 2012, 38, 87–94. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef] [PubMed]
- Woolfit, M.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; Walker, T.; Riegler, M.; Seleznev, A.; Popovici, J.; Rancès, E.; Wee, B.A.; Pavlides, J.; et al. Genomic evolution of the pathogenic Wolbachia strain, wMelPop. Genome Biol. Evol. 2013, 5, 2189–2204. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Ross, P.A.; Rašić, G. Wolbachia strains for disease control: Ecological and evolutionary considerations. Evol. Appl. 2015, 8, 751–768. [Google Scholar] [CrossRef]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [Green Version]
- Rancès, E.; Ye, Y.H.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, M.; Lu, G.; Torres, S.; Edmonds, J.H.; Kay, B.H.; Khromykh, A.A.; Asgari, S. Effect of Wolbachia on replication of west nile virus in a mosquito cell cine and adult mosquitoes. J. Virol. 2013, 87, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; le Nguyen, H.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S.; et al. Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors 2015, 8, 563. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Iturbe-Ormaetxe, I.; Callahan, A.G.; Phillips, B.L.; Billington, K.; Axford, J.K.; Montgomery, B.; Turley, A.P.; O’Neill, S.L. Stability of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations. PLoS Negl. Trop. Dis. 2014, 8, e3115. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled deployment of Wolbachia to protect the community from Aedes transmitted arboviruses. Gates Open Res. 2018, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.D.A.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-de-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Tantowijoyo, W.; Andari, B.; Arguni, E.; Budiwati, N.; Nurhayati, I.; Fitriana, I.; Ernesia, I.; Daniwijaya, E.W.; Supriyati, E.; Yusdiana, D.H.; et al. Stable establishment of wMel Wolbachia in Aedes aegypti populations in Yogyakarta, Indonesia. PLoS Negl. Trop. Dis. 2020, 14, e0008157. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Endersby, N.M.; Yeap, H.L.; Hoffmann, A.A. Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 2014, 91, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axford, J.K.; Callahan, A.G.; Hoffmann, A.A.; Yeap, H.L.; Ross, P.A. Fitness of wAlbB Wolbachia infection in Aedes aegypti: Parameter estimates in an outcrossed background and potential for population invasion. Am. J. Trop. Med. Hyg. 2016, 94, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Joubert, D.A.; Walker, T.; Carrington, L.B.; de Bruyne, J.T.; Kien, D.H.T.; Hoang, N.L.T.; Chau, N.V.V.; Iturbe-Ormaetxe, I.; Simmons, C.P.; O’Neill, S.L. Establishment of a Wolbachia superinfection in Aedes aegypti mosquitoes as a potential approach for future resistance management. PLoS Pathog. 2016, 12, e1005434. [Google Scholar] [CrossRef] [Green Version]
- Turley, A.P.; Moreira, L.A.; O’Neill, S.L.; McGraw, E.A. Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl. Trop. Dis. 2009, 3, e516. [Google Scholar] [CrossRef]
- McMeniman, C.J.; O’Neill, S.L. A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl. Trop. Dis. 2010, 4, e748. [Google Scholar] [CrossRef] [Green Version]
- Yeap, H.L.; Mee, P.; Walker, T.W.; Weeks, A.R.; O’Neill, S.L.; Johnson, P.R.S.; Ritchie, S.A.; Richardson, K.M.; Doig, C.J.; Endersby, N.M.; et al. Dynamics of the “Popcorn” Wolbachia Infection in Outbred Aedes aegypti Informs Prospects for Mosquito Vector Control. Genetics 2011, 186, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Lau, M.J.; Endersby-Harshman, N.M.; Axford, J.K.; Ritchie, S.A.; Hoffmann, A.A.; Ross, P.A. Measuring the host-seeking ability of Aedes aegypti destined for field release. Am. J. Trop. Med. Hyg. 2020, 102, 223–231. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Belinato, T.A.; Gesto, J.S.M.; Martins, A.J.; Bruno, R.V.; Moreira, L.A. Embryonic development and egg viability of wMel-infected Aedes aegypti. Parasites Vectors 2019, 12, 211. [Google Scholar] [CrossRef]
- Christophers, S.R. Aedes Aegypti (L.) The Yellow Fever Mosquito: Its Life History, Bionomics and Structure; Cambridge University Press: Cambridge, UK, 1960; pp. 131–262. [Google Scholar]
- Diniz, D.F.A.; de Albuquerque, C.M.R.; Oliva, L.O.; de Melo-Santos, M.A.V.; Ayres, C.F.J. Diapause and quiescence: Dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasites Vectors 2017, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Vargas, H.C.M.; Farnesi, L.C.; Martins, A.J.; Valle, D.; Rezende, G.L. Serosal cuticle formation and distinct degrees of desiccation resistance in embryos of the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus. J. Insect Physiol. 2014, 62, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C.; McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; et al. Phylogenomics of the reproductive Parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef]
- Lindsey, A.R.I.; Bhattacharya, T.; Newton, I.L.G.; Hardy, R.W. Conflict in the intracellular lives of endosymbionts and viruses: A mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses 2018, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, N.E.; Gerdtzen, Z.P.; Olivera-Nappa, Á.; Salgado, J.C.; Conca, C. A systems biology approach for studying Wolbachia metabolism reveals points of interaction with its host in the context of arboviral infection. PLoS Negl. Trop. Dis. 2019, 13, e0007678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krafsur, A.M.; Ghosh, A.; Brelsfoard, C.L. Phenotypic Response of in a Cell-Free Medium. Microorganisms 2020, 8, 1060. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Tharanathan, R.N.; Weckesser, J. Analysis of lipopolysaccharides of gram-negative bacteria. Methods Microbiol. 1985, 18, 157–207. [Google Scholar] [CrossRef]
- Lam, H.; Oh, D.-C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-amino Acids Govern Stationary Phase Cell Wall Re-Modeling in Bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef] [Green Version]
- Hansen, I.A.; Attardo, G.M.; Rodriguez, S.D.; Drake, L.L. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front. Physiol. 2014, 5, 103. [Google Scholar] [CrossRef] [Green Version]
- Mirth, C.K.; Alves, A.N.; Piper, M.D.W. Turning food into eggs: Insights from nutritional biology and developmental physiology of Drosophila. Curr. Opin. Insect Sci. 2019, 31, 49–57. [Google Scholar] [CrossRef]
- Caragata, E.P. The Role of Resource Competition in the Wolbachia-Host Relationship. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2012. [Google Scholar]
- Molloy, J.C.; Sommer, U.; Viant, M.R.; Sinkins, S.P. Wolbachia modulates lipid metabolism in Aedes albopictus mosquito cells. Appl. Environ. Microbiol. 2016, 82, 3109–3120. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.; Islam, M.N.; Ye, Y.H.; Chotiwan, N.; Graham, B.; Belisle, J.T.; Kouremenos, K.A.; Dayalan, S.; Tull, D.L.; Klatt, S.; et al. Dengue virus dominates lipid metabolism modulations in Wolbachia-coinfected Aedes aegypti. Commun. Biol. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rancès, E.; O’Neill, S.L.; McGraw, E.A. Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb. Ecol. 2014, 67, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, V.; Stainton, K.; Rainey, S.M.; Ant, T.H.; Dowle, A.A.; Larson, T.; Hester, S.; Charles, P.D.; Thomas, B.; Sinkins, S.P. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 2017, 8, 526. [Google Scholar] [CrossRef]
- Manokaran, G.; Flores, H.A.; Dickson, C.T.; Narayana, V.K.; Kanojia, K.; Dayalan, S.; Tull, D.; McConville, M.J.; Mackenzie, J.M.; Simmons, C.P. Modulation of acyl-carnitines, the broad mechanism behind Wolbachia -mediated inhibition of medically important flaviviruses in Aedes aegypti. Proc. Natl. Acad. Sci. USA 2020, 117, 24475–24483. [Google Scholar] [CrossRef] [PubMed]
- Newton, I.L.G.; Rice, D.W. The Jekyll and Hyde symbiont: Could Wolbachia be a nutritional mutualist? J. Bacteriol. 2019. [Google Scholar] [CrossRef]
- Gonzales, K.K.; Hansen, I.A. Artificial diets for mosquitoes. Int. J. Environ. Res. Public Health 2016, 13, 1267. [Google Scholar] [CrossRef] [Green Version]
- Vogel, K.J.; Brown, M.R.; Strand, M.R. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2015, 112, 5057–5062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.R.; Clark, K.D.; Gulia, M.; Zhao, Z.; Garczynski, S.F.; Crim, J.W.; Suderman, R.J.; Strand, M.R. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2008, 105, 5716–5721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klowden, M.J. Distention-mediated egg maturation in the mosquito, Aedes aegypti. J. Insect Physiol. 1987, 33, 83–87. [Google Scholar] [CrossRef]
- Attardo, G.M.; Hansen, I.A.; Shiao, S.-H.; Raikhel, A.S. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. J. Exp. Biol. 2006, 209, 3071–3078. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, A.R.; Laws, K.M.; Drummond-Barbosa, D. Adipocyte amino acid sensing controls adult germline stem cell number via the amino acid response pathway and independently of Target of Rapamycin signaling in Drosophila. Development 2014, 141, 4479–4488. [Google Scholar] [CrossRef] [Green Version]
- Valzania, L.; Mattee, M.T.; Strand, M.R.; Brown, M.R. Blood feeding activates the vitellogenic stage of oogenesis in the mosquito Aedes aegypti through inhibition of glycogen synthase kinase 3 by the insulin and TOR pathways. Dev. Biol. 2019. [Google Scholar] [CrossRef]
- Vital, W.; Rezende, G.L.; Abreu, L.; Moraes, J.; Lemos, F.J.; Vaz, I.D.S.; Logullo, C. Germ band retraction as a landmark in glucose metabolism during Aedes aegypti embryogenesis. BMC Dev. Biol. 2010, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Nagy, L.; Grbić, M. Embryogenesis. In Encyclopedia of Insects; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 316–320. [Google Scholar]
- Farnesi, L.C.; Menna-Barreto, R.F.S.; Martins, A.J.; Valle, D.; Rezende, G.L. Physical features and chitin content of eggs from the mosquito vectors Aedes aegypti, Anopheles aquasalis and Culex quinquefasciatus: Connection with distinct levels of resistance to desiccation. J. Insect Physiol. 2015, 83, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K.; Ohmori, D.; Yamakura, F.; Suzuki, K. Changes in free amino acid concentration in the hemolymph of the female Culex pipiens pallens (Diptera: Culicidae), after a blood meal. J. Med. Entomol. 1990, 27, 302–308. [Google Scholar] [CrossRef]
- Uchida, K.; Oda, T.; Matsuoka, H.; Moribayashi, A.; Ohmori, D.; Eshita, Y.; Fukunaga, A. Induction of oogenesis in mosquitoes (Diptera: Culicidae) by infusion of the hemocoel with amino acids. J. Med. Entomol. 2001, 38, 572–575. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Blanc, E.; Leitão-Gonçalves, R.; Yang, M.; He, X.; Linford, N.J.; Hoddinott, M.P.; Hopfen, C.; Soultoukis, G.A.; Niemeyer, C.; et al. A holidic medium for Drosophila melanogaster. Nat. Methods 2014, 11, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Drummond-Barbosa, D.; Spradling, A.C. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev. Biol. 2001, 231, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat metabolism in insects. Ann. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Tennessen, J.M.; Bertagnolli, N.M.; Evans, J.; Sieber, M.H.; Cox, J.; Thummel, C.S. Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis. G3 Genes Genomes Genet. 2014, 4, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.; Block, K. The absence of sterol synthesis in insects. J. Biol. Chem. 1959, 234, 2578–2582. [Google Scholar]
- Rivera-Pérez, C.; Clifton, M.E.; Noriega, F.G. How micronutrients influence the physiology of mosquitoes. Curr. Opin. Insect Sci. 2017, 23, 112–117. [Google Scholar] [CrossRef]
- Behmer, S.T.; Nes, W.D. Insect Sterol Nutrition and Physiology: A Global Overview; Academic Press: Waltham, MA, USA, 2003; Volume 31, ISBN 0120242311. [Google Scholar]
- Merritt, R.W.; Dadd, R.H.; Walker, E.D. Feeding behaviour, natural food, and nutritional relationships of larval mosquitoes. Ann. Rev. Entomol. 1992, 37, 349–374. [Google Scholar] [CrossRef]
- Feldlaufer, M.F.; Weirich, G.F.; Imberski, R.B.; Svoboda, J.A. Ecdysteroid production in Drosophila melanogaster reared on defined diets. Insect Biochem. Mol. Biol. 1995, 25, 709–712. [Google Scholar] [CrossRef]
- Talyuli, O.A.C.; Bottino-Rojas, V.; Taracena, M.L.; Soares, A.L.M.; Oliveira, J.H.M.; Oliveira, P.L. The use of a chemically defined artificial diet as a tool to study Aedes aegypti physiology. J. Insect Physiol. 2015, 83, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Briegel, H.; Hefti, M.; DiMarco, E. Lipid metabolism during sequential gonotrophic cycles in large and small female Aedes aegypti. J. Insect Physiol. 2002, 48, 547–554. [Google Scholar] [CrossRef]
- Sieber, M.H.; Spradling, A.C. Steroid signaling establishes a female metabolic state and regulates SREBP to control oocyte lipid accumulation. Curr. Biol. 2015, 25, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terradas, G.; McGraw, E.A. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr. Opin. Insect Sci. 2017, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Frentiu, F.D. Lipids and Pathogen Blocking by Wolbachia. Trends Parasitol. 2017, 33, 916–917. [Google Scholar] [CrossRef]
- Cho, K.O.; Kim, G.W.; Lee, O.K. Wolbachia bacteria reside in host Golgi-related vesicles whose position is regulated by polarity proteins. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [Green Version]
- Carro, A.C.; Damonte, E.B. Requirement of cholesterol in the viral envelope for dengue virus infection. Virus Res. 2013, 174, 78–87. [Google Scholar] [CrossRef]
- Cosgrove, J.B.; Wood, R.J. Effects of variations in a formulated protein meal on the fecundity and fertility of female mosquitoes. Med. Vet. Entomol. 1996, 10, 260–264. [Google Scholar] [CrossRef]
- Gonzales, K.K.; Tsujimoto, H.; Hansen, I.A. Blood serum and BSA, but neither red blood cells nor hemoglobin can support vitellogenesis and egg production in the dengue vector Aedes aegypti. PeerJ 2015, 3, e938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Kohlhepp, P.; Geiser, D.; Frasquillo, M.D.C.; Vazquez-Moreno, L.; Winzerling, J.J. Fate of blood meal iron in mosquitoes. J. Insect Physiol. 2007, 53, 1169–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutra, H.L.C.; Rodrigues, S.L.; Mansur, S.B.; de Oliveira, S.P.; Caragata, E.P.; Moreira, L.A. Development and physiological effects of an artificial diet for Wolbachia-infected Aedes aegypti. Sci. Rep. 2017, 7, 15687. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, J.C.; Cass, B.N.; Riegler, M.; Witsenburg, J.J.; Iturbe-Ormaetxe, I.; McGraw, E.A.; O’Neill, S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009, 5, e1000368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, N.; Voronin, D.; Charif, D.; Mavingui, P.; Mollereau, B.; Vavre, F. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef]
| Wolbachia Strain | Fecundity | Larval Development | Adult Female Longevity | Blood Feeding Success | Egg Longevity |
|---|---|---|---|---|---|
| wMel | Minor or no negative impact [32,33,40,47] | No negative impact [32] | No negative impact [15,33] | No negative impact [51] | Decreased [15,47,51] or no negative impact [33] |
| wAlbB | No negative impact [33,46,47] | No negative impact [46] | Minor or no negative impact [33,46,47] | No negative impact [51] | Decreased [33,46,47] or no negative impact [33] |
| wMelPop-CLA | Decreased (age associated) [32,49,50] | Survival not impacted. Delay in male pupation and female 4th instar larval development [49] | Decreased [13,50] | Decreased [48], exacerbated with age [49] | Decreased [32,49,50] |
| Essential | Semi-Essential | Non-Essential |
|---|---|---|
| Leucine | Cysteine | Tyrosine |
| Tryptophan | Glycine | Aspartic acid |
| Methionine | Isoleucine | Serine |
| Valine | Proline | |
| Histidine | Glutamine | |
| Lysine | Alanine | |
| Phenylalanine | Glutamic acid | |
| Arginine | ||
| Asparagine | ||
| Threonine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allman, M.J.; Fraser, J.E.; Ritchie, S.A.; Joubert, D.A.; Simmons, C.P.; Flores, H.A. Wolbachia’s Deleterious Impact on Aedes aegypti Egg Development: The Potential Role of Nutritional Parasitism. Insects 2020, 11, 735. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110735
Allman MJ, Fraser JE, Ritchie SA, Joubert DA, Simmons CP, Flores HA. Wolbachia’s Deleterious Impact on Aedes aegypti Egg Development: The Potential Role of Nutritional Parasitism. Insects. 2020; 11(11):735. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110735
Chicago/Turabian StyleAllman, Megan J., Johanna E. Fraser, Scott A. Ritchie, D. Albert Joubert, Cameron P. Simmons, and Heather A. Flores. 2020. "Wolbachia’s Deleterious Impact on Aedes aegypti Egg Development: The Potential Role of Nutritional Parasitism" Insects 11, no. 11: 735. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110735





