Mediterranean Fruit Fly Ceratitis capitata (Diptera: Tephritidae) Eggs and Larvae Responses to a Low-Oxygen/High-Nitrogen Atmosphere
Abstract
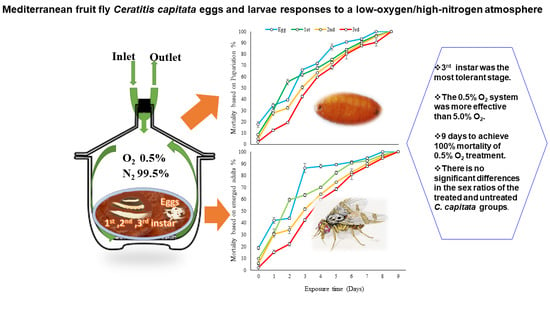
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture
2.2. Low-Oxygen Treatment Facility
2.3. Low-Oxygen Treatment
2.4. Statistical Analysis
3. Results
Sex Ratios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, M.C.; Heppner, J.B.; Woodruff, R.E.; Weems, H.V.; Steck, G.J.; Fasulo, T.R. Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann)(Insecta: Diptera: Tephritidae); Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Entomology Circulars: Gainesville, FL, USA, 2001. [Google Scholar]
- Woods, B.; Lacey, I.B.; Brockway, C.A.; Christine, P.J. Hosts of Mediterranean fruit fly Ceratitis capitata (Wiedemann)(Diptera: Tephritidae) from Broome and the Broome Peninsula, Western Australia. Aust. J. Entomol. 2005, 44, 437–441. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Sabrina, B.; Gasperi, G.; Guglielmino, C.R. Globalization and fruitfly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.; De Lima CP., F. Field evaluation of female attractants for monitoring Ceratitis capitata (Diptera: Tephritidae) under a range of climatic conditions and population levels in Western Australia. J. Econ. Entomol. 2002, 95, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Al-Behadili, F.J.M.; Vineeta, B.; Junxi, L.; Penghao, W.; Miyuki, T.; Manjree, A.; Yonglin, R.; Wei, L. Cold response of the Mediterranean fruit fly (Ceratitis capitata) on a lab diet. Insects 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villers, P.; Navarro, S.; DeBruin, T. Development of hermetic storage technology in sealed flexible storage structures. In Proceedings of the 8th International Conference on Controlled Atmosphere and Fumigation in Stored Products, Chengdu, China, 21–26 September 2008; pp. 21–26. [Google Scholar]
- Mditshwa, A.; Olaniyi, A.F.; Umezuruike, U.O. Recent developments on dynamic controlled atmosphere storage of apples—A review. Food Packag. Shelf Life 2018, 16, 59–68. [Google Scholar] [CrossRef]
- Frazier, M.R.; Woods, H.A.; Jon, F. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol. Biochem. Zool. 2001, 74, 641–650. [Google Scholar] [CrossRef]
- Kloek, G.P. Oxygen levels safe for continued reproduction of Drosophila in normal and hypobaric atmospheres. Aviat. Space Environ. Med. 1979, 50, 1126–1128. [Google Scholar]
- De Lima, C.P.F. Airtight storage: Principle and practice. In Food Preservation by Modified Atmospheres; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 9–19. [Google Scholar]
- Chiappini, E.; Paola, M.; Piero, C. Mortality of Tribolium confusum J. du Val (Coleoptera: Tenebrionidae) in controlled atmospheres at different oxygen percentages. J. Stored Prod. Res. 2009, 45, 10–13. [Google Scholar] [CrossRef]
- Agnello, A.M.; Steve, M.; Spangler, Eve, S.M.; Tracy, H.; David, P.K. Effect of high-carbon dioxide atmospheres on infestations of apple maggot (Diptera: Tephritidae) in apples. J. Econ. Entomol. 2002, 95, 520–526. [Google Scholar] [CrossRef]
- Gorny, J.R. Modified atmosphere packaging in fresh-cut revolution. Perish. Handl. Newsl. 1997, 90, 4–5. [Google Scholar]
- Herner, R.C. High CO2 effects on plant organs. In Postharvest Physiology of Vegetables; M Dekker: New York, NY, USA, 1987; pp. 239–253. [Google Scholar]
- Chen, P.M.; Olsen, K.L.; Meheriuk, M. Effect of low-oxygen atmosphere on storage scald and quality preservation of «Delicious» apples. J. Am. Soc. Hortic. Sci. 1985, 110, 16–20. [Google Scholar]
- Mathooko, F.M. Regulation of respiratory metabolism in fruits and vegetables by carbon dioxide. Postharvest Biol. Technol. 1996, 9, 247–264. [Google Scholar] [CrossRef]
- Ke, D.; Kader, A.A. Potential of controlled atmospheres for postharvest insect disinfestation of fruits and vegetables. Postharvest News Inf. 1992, 3, 31N–37N. [Google Scholar]
- Cao, Y.; Kangkang, X.; Xiaoye, Z.; Yu, B.; Wenjia, Y.; Can, L. Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol. 2019, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, F.; Michael, H.D.; Jocelyn, S. The scaling of carbon dioxide release and respiratory water loss in flying fruit flies (Drosophila spp.). J. Exp. Biol. 2000, 203, 1613–1624. [Google Scholar]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Steiner, R.W. Carbon dioxide’s expanding role. Chem. Eng. 1993, 100, 114. [Google Scholar]
- Smilanick, J.L.; Fouse, D.C. Quality of nectarines stored in insecticidal low-O2 atmospheres at 5 and 15C. J. Am. Soc. Hortic. Sci. 1989, 114, 431–436. [Google Scholar]
- Yahia, E.M.; Marisela, R.; Omar, H. Responses of papaya to short-term insecticidal oxygen atmosphere. J. Am. Soc. Hortic. Sci. 1992, 117, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, T.; Akiko, T.; Shino, M.; Toshiyuki, A.; Masahiro, T.; Yoshie, M. Water-soaked symptom of’Andesu’netted melon fruit does not develop under anaerobic nitrogen atmospheres during ripening. Plant Growth Regul. 2002, 38, 7–14. [Google Scholar] [CrossRef]
- Yahia, E.M. Modified and controlled atmospheres for tropical fruits. Hortic. Rev. Westport N. Y. 1998, 22, 123–183. [Google Scholar]
- Hanlon, G.; Vinod, D.; Nancie, R.; Shin, M. Dynamic system for nitrogen anoxia of large museum objects: A pest eradication case study. In Wooden Artifacts Group: Specialty Session, 4 June 1993, AIC Annual Meeting, Denver, Colorado; AIC Wooden Artifacts Group: Washington, DC, USA, 1993; pp. 52–70. [Google Scholar]
- Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.; Parker, A.G.; Caravantes, L.A.; Rivera, C.; Araújo, A.S.; Maxwell, F.; et al. Modified Atmosphere Does Not Reduce the Efficacy of Phytosanitary Irradiation Doses Recommended for Tephritid Fruit Flies. Insects 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Follett, P.A.; Neven, L.G. Phytosanitary irradiation: Does modified atmosphere packaging or controlled atmosphere storage creating a low oxygen environment threaten treatment efficacy? Radiat. Phys. Chem. 2020, 173, 108874. [Google Scholar] [CrossRef]
- Burg, S.P. Postharvest Physiology and Hypobaric Storage of Fresh Produce; CABI Pub: Wallingford, UK; Oxon, UK; Cambridge, MA, USA, 2004; 654p, ISBN 0-85199-267-6. [Google Scholar]
- Wang, F.; Chambi, C.; Li, Z.; Huang, C.; Ma, Y.; Li, C.; Tian, X.; Sangija, F.; Ntambo, M.S.; Kankonda, O.M.; et al. Influence of supplemental protein on the life expectancy and reproduction of the Chinese citrus fruit fly, Bactrocera minax (Enderlein)(Tetradacus minax)(Diptera: Tephritidae). J. Insect Sci. 2018, 18, 25. [Google Scholar] [CrossRef]
- Tanaka, N.; Steiner, L.; Ohinata, K.; Okamoto, R. Low-cost larval rearing medium for mass production of oriental and Mediterranean fruit flies. J. Econ. Entomol. 1969, 62, 967–968. [Google Scholar] [CrossRef]
- Frías, D.; Hernández-Ortiz, V.; Vaccaro, N.C.; Bartolucci, A.F.; Salles, L.A.S. Comparative Morphology of Immature Stages of Some Frugivorous Species of Fruit Flies (Diptera:Tephritidae). Israel J. Entomol. 2006, 35, 423–457. [Google Scholar]
- Püntener, W. Manual for Field Trials in Plant Protection; Ciba-Geigy, Limited: Basle, Switzerland, 1981. [Google Scholar]
- Maekawa, S.; Elert, K. The Use of Oxygen-Free Environments in the Control of Museum Insect Pests; Getty Publications: Los Angeles, CA, USA, 2003. [Google Scholar]
- Van Voorhies, W.A. Metabolic function in Drosophila melanogaster in response to hypoxia and pure oxygen. J. Exp. Biol. 2009, 212, 3132–3141. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Williams, S.B.; Baributsa, D.; Murdock, L.L. Hypoxia treatment of Callosobruchus maculatus Females and its effects on reproductive output and development of progeny following exposure. Insects 2016, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Yahia, E.M.; Ortega-Zaleta, D. Mortality of eggs and third instar larvae of Anastrepha ludens and A. obliqua with insecticidal controlled atmospheres at high temperatures. Postharvest Biol. Technol. 2000, 20, 295–302. [Google Scholar] [CrossRef]
- Shellie, K.C. Vine and berry response of Merlot (Vitis vinifera L.) to differential water stress. Am. J. Enol. Vitic. 2006, 57, 514–518. [Google Scholar]
- Kingsolver, J.G.; Arthur Woods, H.; Buckley, L.B.; Potter, K.A.; MacLean, H.J.; Higgins, J.K. Complex life cycles and the responses of insects to climate change. Integr. Comp. Biol. 2011, 51, 719–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callier, V.; Hand, S.C.; Campbell, J.B.; Biddulph, T.; Harrison, J.F. Developmental changes in hypoxic exposure and responses to anoxia in Drosophila melanogaster. J. Exp. Biol. 2015, 218, 2927–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.F.; Haddad, G.G. Effects of oxygen on growth and size: Synthesis of molecular, organismal, and evolutionary studies with Drosophila melanogaster. Annu. Rev. Physiol. 2011, 73, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Hetz, S.K.; Bradley, T.J. Insects breathe discontinuously to avoid oxygen toxicity. Nature 2005, 433, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Lighton, J.R.; Schilman, P.E. Oxygen reperfusion damage in an insect. PLoS ONE 2007, 2, e1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fišer, C. Life histories. In Encyclopedia of Caves; Academic Press: New York, NY, USA, 2019; pp. 652–657. [Google Scholar]
- Skalski, J.R.; Ryding, K.E.; Millspaugh, J. Wildlife Demography: Analysis of Sex, Age, and Count Data; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Walder, J.M.M.; Calkins, C.O. Effects of gamma radiation on the sterility and behavioral quality of the Caribbean fruit fly, Anastrepha suspensa (Loew) (Diptera: Tephritidae). Sci. Agrícola 1993, 50, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Sapir, Y.; Mazer, S.; Holzapfel, C.; Erik, J.; Brian, F. Sex ratio. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Moffitt, H.R.; Albano, D.J. Effects of commercial fruit storage on stages of the codling moth. J. Econ. Entomol. 1972, 65, 770–773. [Google Scholar] [CrossRef]




| Treatment | Stage | Mortality Based on Pupariation | Mortality Based on Emerged Adults | ||||
|---|---|---|---|---|---|---|---|
| LT 50 | LT 90 | LT 99 | LT 50 | LT 90 | LT 99 | ||
| (95% *CL) | (95% CL) | (95% CL) | (95% CL) | (95% CL) | (95% CL) | ||
| 0.5% O2 | Egg | 2.8 (2.6–3.1) | 6.0 (5.6–6.4) | 8.6 (8.0–9.3) | 2.1 (1.3–2.8) | 5.2 (4.3–6.6) | 8.5 (6.9–11.5) |
| 1st | 2.8 (2.3–3.3) | 6.5 (5.9–7.4) | 9.5 (8.5–11.1) | 2.6 (2.1–3.0) | 6.0 (5.4–6.8) | 8.8 (7.8–10.2) | |
| 2nd | 3.2 (2.9–3.4) | 6.7 (6.3–7.1) | 9.5 (8.9–10.3) | 3.3 (3.0–3.5) | 6.7 (6.3–7.1) | 9.5 (8.9–10.2) | |
| 3rd | 3.8 (3.5–4.0) | 7.0 (6.6–7.4) | 10.4 (9.7–11.3) | 3.7 (3.5–4.0) | 6.9 (6.6–7.4) | 9.6 (9.0–10.2) | |
| 5.0% O2 | Egg | 5.2 (4.6–5.7) | 9.5 (8.5–10.9) | 14.1 (12.4–16.8) | 4.9 (4.5–5.2) | 9.4 (8.8–10.1) | 14.3 (13.2–15.7) |
| 1st | 5.6 (5.3–6.0) | 10.4 (9.7–11.3) | 15.6 (14.4–17.3) | 5.3 (5.0–5.6) | 10.2 (9.5–11.0) | 15.2 (14.2–17.1) | |
| 2nd | 6.8 (6.3–7.4) | 13.1 (11.9–14.7) | 18.2 (16.4–20.7) | 6.0 (5.3–6.9) | 11.8 (10.3–14.3) | 16.6 (14.1–20.6) | |
| 3rd | 9.5 (8.6–10.7) | 16.8 (14.8–19.7) | 22.8 (19.8–27.1) | 7.7 (7.2–8.4) | 13.9 (12.6–15.7) | 19.0 (17.0–21.7) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Behadili, F.J.M.; Agarwal, M.; Xu, W.; Ren, Y. Mediterranean Fruit Fly Ceratitis capitata (Diptera: Tephritidae) Eggs and Larvae Responses to a Low-Oxygen/High-Nitrogen Atmosphere. Insects 2020, 11, 802. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110802
Al-Behadili FJM, Agarwal M, Xu W, Ren Y. Mediterranean Fruit Fly Ceratitis capitata (Diptera: Tephritidae) Eggs and Larvae Responses to a Low-Oxygen/High-Nitrogen Atmosphere. Insects. 2020; 11(11):802. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110802
Chicago/Turabian StyleAl-Behadili, Farhan J.M., Manjree Agarwal, Wei Xu, and Yonglin Ren. 2020. "Mediterranean Fruit Fly Ceratitis capitata (Diptera: Tephritidae) Eggs and Larvae Responses to a Low-Oxygen/High-Nitrogen Atmosphere" Insects 11, no. 11: 802. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110802