Influence of Temperature on the Life-Cycle Dynamics of Aedes albopictus Population Established at Temperate Latitudes: A Laboratory Experiment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Colony
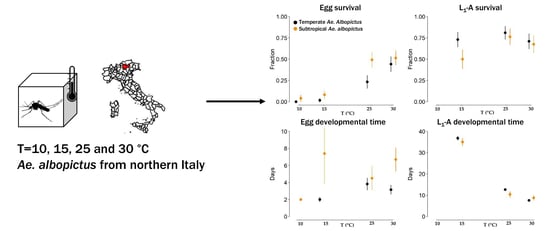
2.2. Experimental Conditions
2.3. Egg-Hatching Experiment
2.4. Larval Survival and Developmental Time
2.5. Adult Longevity and Gonotrophic Cycle
2.6. Statistical Analysis
3. Results
3.1. Survivorship and Length of Development of Eggs and Immature Stages
3.2. Adult Life Expectancies
3.3. Gonotrophic Cycles and Egg Laying
3.4. Population Dynamics Simulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Bayesian Fitting of the Posterior Distributions of Life History Parameters








Appendix A.2. Adult Survival
Appendix A.3. Population Dynamics Simulation


References
- European Centre for Disease Prevention and Control. Guidelines for the Surveillance of Invasive Mosquitoes in Europe; ECDC: Stockholm, Sweden, 2012; ISBN 978-92-9193-378-5.
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Van Bortel, W. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control Mosquito Maps. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/mosquito-maps (accessed on 1 September 2020).
- Gasperi, G.; Bellini, R.; Malacrida, A.R.; Crisanti, A.; Dottori, M.; Aksoy, S. A New Threat Looming over the Mediterranean Basin: Emergence of Viral Diseases Transmitted by Aedes albopictus Mosquitoes. PLoS Negl. Trop. Dis. 2012, 6, e1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira-dos-Santos, T.; Roiz, D.; Lourenço-de-Oliveira, R.; Paupy, C. A Systematic Review: Is Aedes albopictus an Efficient Bridge Vector for Zoonotic Arboviruses? Pathogens 2020, 9, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Venturi, G.; Luca, M.D.; Fortuna, C.; Remoli, M.E.; Riccardo, F.; Severini, F.; Toma, L.; Manso, M.D.; Benedetti, E.; Caporali, M.G.; et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Eurosurveillance 2017, 22, 17–00646. [Google Scholar] [CrossRef] [Green Version]
- Delisle, E.; Rousseau, C.; Broche, B.; Leparc-Goffart, I.; L’Ambert, G.; Cochet, A.; Prat, C.; Foulongne, V.; Ferré, J.B.; Catelinois, O.; et al. Chikungunya outbreak in Montpellier, France, September to October 2014. Eurosurveillance 2015, 20, 21108. [Google Scholar] [CrossRef] [Green Version]
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’Ambert, G.; Grard, G.; et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Eurosurveillance 2019, 24, 1900655. [Google Scholar] [CrossRef] [Green Version]
- Gjenero-Margan, I.; Aleraj, B.; Krajcar, D.; Lesnikar, V.; Klobučar, A.; Pem-Novosel, I.; Kurečić-Filipović, S.; Komparak, S.; Martić, R.; Đuričić, S.; et al. Autochthonous dengue fever in Croatia, August–September 2010. Eurosurveillance 2011, 16, 19805. [Google Scholar] [CrossRef]
- Lazzarini, L.; Barzon, L.; Foglia, F.; Manfrin, V.; Pacenti, M.; Pavan, G.; Rassu, M.; Capelli, G.; Montarsi, F.; Martini, S.; et al. First autochthonous dengue outbreak in Italy, August 2020. Eurosurveillance 2020, 25, 2001606. [Google Scholar] [CrossRef]
- Marchand, E.; Prat, C.; Jeannin, C.; Lafont, E.; Bergmann, T.; Flusin, O.; Rizzi, J.; Roux, N.; Busso, V.; Deniau, J.; et al. Autochthonous case of dengue in France, October 2013. Eurosurveillance 2013, 18, 20661. [Google Scholar] [CrossRef] [Green Version]
- Monge, S.; García-Ortúzar, V.; López Hernández, B.; Lopaz Pérez, M.Á.; Delacour-Estrella, S.; Sánchez-Seco, M.P.; Fernández Martinez, B.; García San Miguel, L.; García-Fulgueiras, A.; Sierra Moros, M.J. Characterization of the first autochthonous dengue outbreak in Spain (August–September 2018). Acta Trop. 2020, 205, 105402. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit. Vectors 2014, 7, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caminade, C.; Medlock, J.M.; Ducheyne, E.; McIntyre, K.M.; Leach, S.; Baylis, M.; Morse, A.P. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: Recent trends and future scenarios. J. R. Soc. Interface 2012, 9, 2708–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, D.; Thomas, S.M.; Neteler, M.; Tjaden, N.B.; Beierkuhnlein, C. Climatic suitability of Aedes albopictus in Europe referring to climate change projections: Comparison of mechanistic and correlative niche modelling approaches. Eurosurveillance 2014, 19, 20696. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Delatte, H.; Gimonneau, G.; Triboire, A.; Fontenille, D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J. Med. Entomol. 2009, 46, 33–41. [Google Scholar] [CrossRef]
- Loetti, V.; Schweigmann, N.; Burroni, N. Development rates, larval survivorship and wing length of Culex pipiens (Diptera: Culicidae) at constant temperatures. J. Nat. Hist. 2011, 45, 2203–2213. [Google Scholar] [CrossRef]
- Marini, G.; Arnoldi, D.; Baldacchino, F.; Capelli, G.; Guzzetta, G.; Merler, S.; Montarsi, F.; Rizzoli, A.; Rosà, R. First report of the influence of temperature on the bionomics and population dynamics of Aedes koreicus, a new invasive alien species in Europe. Parasit. Vectors 2019, 12, 524. [Google Scholar] [CrossRef]
- Reinhold, J.M.; Lazzari, C.R.; Lahondère, C. Effects of the Environmental Temperature on Aedes aegypti and Aedes albopictus Mosquitoes: A Review. Insects 2018, 9, 158. [Google Scholar] [CrossRef] [Green Version]
- Reuss, F.; Wieser, A.; Niamir, A.; Bálint, M.; Kuch, U.; Pfenninger, M.; Müller, R. Thermal experiments with the Asian bush mosquito (Aedes japonicus japonicus) (Diptera: Culicidae) and implications for its distribution in Germany. Parasit. Vectors 2018, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Alto, B.W.; Juliano, S.A. Precipitation and Temperature Effects on Populations of Aedes albopictus (Diptera: Culicidae): Implications for Range Expansion. J. Med. Entomol. 2001, 38, 646–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alto, B.W.; Juliano, S.A. Temperature Effects on the Dynamics of Aedes albopictus (Diptera: Culicidae) Populations in the Laboratory. J. Med. Entomol. 2001, 38, 548–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, L.C.C.; de Souza, J.R.B.; de Albuquerque, C.M.R. Eclosion rate, development and survivorship of Aedes albopictus (Skuse) (Diptera: Culicidae) under different water temperatures. Neotrop. Entomol. 2007, 36, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Léger, L.; L’Ambert, G.; Lacour, G.; Foussadier, R.; Besnard, G.; Barré-Cardi, H.; Simard, F.; Fontenille, D. The Spread of Aedes albopictus in Metropolitan France: Contribution of Environmental Drivers and Human Activities and Predictions for a Near Future. PLoS ONE 2015, 10, e0125600. [Google Scholar] [CrossRef] [Green Version]
- Pluskota, B.; Jöst, A.; Augsten, X.; Stelzner, L.; Ferstl, I.; Becker, N. Successful overwintering of Aedes albopictus in Germany. Parasitol. Res. 2016, 115, 3245–3247. [Google Scholar] [CrossRef]
- Brady, O.J.; Johansson, M.A.; Guerra, C.A.; Bhatt, S.; Golding, N.; Pigott, D.M.; Delatte, H.; Grech, M.G.; Leisnham, P.T.; Maciel-de-Freitas, R.; et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit. Vectors 2013, 6, 351. [Google Scholar] [CrossRef] [Green Version]
- Guzzetta, G.; Montarsi, F.; Baldacchino, F.A.; Metz, M.; Capelli, G.; Rizzoli, A.; Pugliese, A.; Rosà, R.; Poletti, P.; Merler, S. Potential Risk of Dengue and Chikungunya Outbreaks in Northern Italy Based on a Population Model of Aedes albopictus (Diptera: Culicidae). PLoS Negl. Trop. Dis. 2016, 10, e0004762. [Google Scholar] [CrossRef]
- Manica, M.; Guzzetta, G.; Poletti, P.; Filipponi, F.; Solimini, A.; Caputo, B.; Torre, A.d.; Rosà, R.; Merler, S. Transmission dynamics of the ongoing chikungunya outbreak in Central Italy: From coastal areas to the metropolitan city of Rome, summer 2017. Eurosurveillance 2017, 22, 17–00685. [Google Scholar] [CrossRef]
- Marini, G.; Guzzetta, G.; Baldacchino, F.; Arnoldi, D.; Montarsi, F.; Capelli, G.; Rizzoli, A.; Merler, S.; Rosà, R. The effect of interspecific competition on the temporal dynamics of Aedes albopictus and Culex pipiens. Parasit. Vectors 2017, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, S.; Mariani, L.; Calvitti, M.; Moretti, R.; Ponti, L.; Chiari, M.; Sperandio, G.; Gilioli, G. Development and calibration of a model for the potential establishment and impact of Aedes albopictus in Europe. Acta Trop. 2020, 202, 105228. [Google Scholar] [CrossRef] [PubMed]
- Poletti, P.; Messeri, G.; Ajelli, M.; Vallorani, R.; Rizzo, C.; Merler, S. Transmission Potential of Chikungunya Virus and Control Measures: The Case of Italy. PLoS ONE 2011, 6, e18860. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; L’Ambert, G.; Lacour, G.; Benoît, R.; Demarchi, M.; Cros, M.; Cailly, P.; Aubry-Kientz, M.; Balenghien, T.; Ezanno, P. A Rainfall- and Temperature-Driven Abundance Model for Aedes albopictus Populations. Int. J. Environ. Res. Public. Health 2013, 10, 1698–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roiz, D.; Rosà, R.; Arnoldi, D.; Rizzoli, A. Effects of Temperature and Rainfall on the Activity and Dynamics of Host-Seeking Aedes albopictus Females in Northern Italy. Vector Borne Zoonotic Dis. 2010, 10, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Rosso, F.; Tagliapietra, V.; Albanese, D.; Pindo, M.; Baldacchino, F.; Arnoldi, D.; Donati, C.; Rizzoli, A. Reduced diversity of gut microbiota in two Aedes mosquitoes species in areas of recent invasion. Sci. Rep. 2018, 8, 16091. [Google Scholar] [CrossRef]
- Gilks, W.R.; Richardson, S.; Spiegelhalter, D. Markov Chain Monte Carlo in Practice; Chapman & Hall: London, UK, 1996; ISBN 0-412-05551-1. [Google Scholar]
- Cunze, S.; Kochmann, J.; Koch, L.K.; Klimpel, S. Aedes albopictus and Its Environmental Limits in Europe. PLoS ONE 2016, 11, e0162116. [Google Scholar] [CrossRef] [Green Version]
- Meteotrentino Meteotrentino. Available online: www.meteotrentino.it (accessed on 31 August 2020).
- Mordecai, E.A.; Caldwell, J.M.; Grossman, M.K.; Lippi, C.A.; Johnson, L.R.; Neira, M.; Rohr, J.R.; Ryan, S.J.; Savage, V.; Shocket, M.S.; et al. Thermal biology of mosquito-borne disease. Ecol. Lett. 2019, 22, 1690–1708. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control. Vector Control with a Focus on Aedes Aegypti and Aedes Albopictus Mosquitoes: Literature Review and Analysis of Information; ECDC: Stockholm, Sweden, 2017; ISBN 978-92-9498-095-3.
- Erguler, K.; Pontiki, I.; Zittis, G.; Proestos, Y.; Christodoulou, V.; Tsirigotakis, N.; Antoniou, M.; Kasap, O.E.; Alten, B.; Lelieveld, J. A climate-driven and field data-assimilated population dynamics model of sand flies. Sci. Rep. 2019, 9, 2469. [Google Scholar] [CrossRef]
- Medley, K.A.; Westby, K.M.; Jenkins, D.G. Rapid local adaptation to northern winters in the invasive Asian tiger mosquito Aedes albopictus: A moving target. J. Appl. Ecol. 2019, 56, 2518–2527. [Google Scholar] [CrossRef]
- Erguler, K.; Smith-Unna, S.E.; Waldock, J.; Proestos, Y.; Christophides, G.K.; Lelieveld, J.; Parham, P.E. Large-Scale Modelling of the Environmentally-Driven Population Dynamics of Temperate Aedes albopictus (Skuse). PLoS ONE 2016, 11, e0149282. [Google Scholar] [CrossRef] [Green Version]
- Neteler, M.; Roiz, D.; Rocchini, D.; Castellani, C.; Rizzoli, A. Terra and Aqua satellites track tiger mosquito invasion: Modelling the potential distribution of Aedes albopictus in north-eastern Italy. Int. J. Health Geogr. 2011, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| T (°C) | Eggs (n) | Egg-L1 | L1 (n) | L1-L2 | L2-L3 | L3-L4 | L4-Pupae | Pupae-Adult | L1-Adult | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | |
| 10 | 120 | 100 | 0 | 0.04 ± 0.02 | 100 | 80 | 0.38 ± 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 120 | 110 | 0.02 ± 0.01 | 0.08 ± 0.03 | 100 | 80 | 0.98 ± 0.01 | 0.89 ± 0.04 | 0.99 ± 0.01 | 0.93 ± 0.03 | 1 | 0.86 ± 004 | 0.98 ± 0.01 | 0.84 ± 0.05 | 0.77 ± 0.04 | 0.83 ± 0.05 | 0.73 ± 0.04 | 0.5 ± 0.06 |
| 25 | 120 | 130 | 0.23 ± 0.04 | 0.49 ± 0.04 | 100 | 80 | 0.97 ± 0.02 | 0.93 ± 0.03 | 0.99 ± 0.01 | 0.95 ± 0.03 | 0.99 ± 0.01 | 0.96 ± 0.02 | 0.99 ± 0.01 | 0.97 ± 0.02 | 0.86 ± 0.04 | 0.94 ± 0.03 | 0.81 ± 0.04 | 0.76 ± 0.05 |
| 30 | 120 | 140 | 0.44 ± 0.05 | 0.51 ± 0.04 | 100 | 80 | 0.97 ± 0.02 | 0.88 ± 0.04 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.98 ± 0.01 | 0.96 ± 0.02 | 0.9 ± 0.03 | 0.91 ± 0.04 | 0.84 ± 0.04 | 0.9 ± 0.04 | 0.71 ± 0.05 | 0.68 ± 0.05 |
| T (°C) | Eggs-L1 | L1-L2 | L2-L3 | L3-L4 | L4-Pupae | Pupae-Adult | L1-Adult | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | TE | ST | |
| 10 | 2.0 ± 0.0 | 37.4 ± 0.7 | ||||||||||||
| 15 | 2.0 ± 0.1 | 7.4 ± 1.8 | 5.8 ± 0.1 | 5.6 ± 0.3 | 5.1 ± 0.2 | 3.3 ± 0.2 | 7.0 ± 0.1 | 4.6 ± 0.2 | 16.7 ± 0.4 | 13.4 ± 0.8 | 10.7 ± 0.3 | 8.7 ± 0.6 | 36.8 ± 0.5 | 35 ± 0.9 |
| 25 | 3.8 ± 0.4 | 4.5 ± 0.7 | 2.5 ± 0.1 | 2.1 ± 0.2 | 2.0 ± 0.1 | 1.2 ± 0.2 | 2.4 ± 0.1 | 1.2 ± 0.1 | 5.0 ± 0.1 | 3.3 ± 0.2 | 3.5 ± 0.1 | 2.7 ± 0.1 | 12.7 ± 0.2 | 10.4 ± 0.7 |
| 30 | 3.2 ± 0.3 | 6.7 ± 0.7 | 2.0 ± 0.0 | 1.4 ± 0.1 | 1.1 ± 0.0 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.2 | 3.5 ± 0.1 | 3.0 ± 0.3 | 1.9 ± 0.0 | 1.9 ± 0.1 | 7.6 ± 0.1 | 8.8 ± 0.6 |
| T (°C) | Mean ± SE (Days) | Range (min–max) (Days) |
|---|---|---|
| 10 | 5.7 ± 0.4 | 2–10 |
| 15 | 27.9 ± 2.0 | 8–45 |
| 25 | 64.5 ± 3.8 | 32–92 |
| 30 | 53.5 ± 4.7 | 14–86 |
| 25 °C | 30 °C | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Duration (Mean ± SE) | Minimum Duration | Number of Eggs (Mean ± SE) | n | Mean | Minimum Duration | Number of Eggs (Mean ± SE) | |
| Cycle 1 | 20 | 4.2 ± 0.1 | 4 | 31.7 ± 2.0 | 20 | 3.1 ± 0.1 | 2 | 55.9 ± 4.0 |
| Cycle 2 | 20 | 4.0 ± 0.0 | 3 | 40.1 ± 2.3 | 12 | 3.3 ± 0.2 | 2 | 55.0 ± 8.2 |
| Cycle 3 | 20 | 3.0 ± 0.0 | 3 | 40.8 ± 2.1 | 3 | 4.3 ± 0.9 | 3 | 60.7 ± 13.2 |
| Cycle 4 | 20 | 4.0 ± 0.0 | 4 | 40.4 ± 2.3 | ||||
| Cycle 5 | 17 | 3.0 ± 0.0 | 3 | 39.4 ± 2.5 | ||||
| Cycle 6 | 16 | 3.6 ± 0.2 | 3 | 31.6 ± 4.7 | ||||
| Cycle 7 | 16 | 3.9 ± 0.1 | 3 | 30.0 ± 3.5 | ||||
| Cycle 8 | 13 | 4.2 ± 0.4 | 3 | 25.4 ± 5.0 | ||||
| Cycle 9 | 9 | 4.1 ± 0.1 | 4 | 29.4 ± 8.3 | ||||
| Cycle 10 | 5 | 4.2 ± 0.2 | 4 | 19.0 ± 0.4 | ||||
| Cycle 11 | 2 | 7.5 ± 2.5 | 5 | 6.0 ± 3.0 | ||||
| Mean | 3.8 ± 0.1 | 34.3 ± 1.2 | 3.3 ± 0.1 | 56.0 ± 3.7 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marini, G.; Manica, M.; Arnoldi, D.; Inama, E.; Rosà, R.; Rizzoli, A. Influence of Temperature on the Life-Cycle Dynamics of Aedes albopictus Population Established at Temperate Latitudes: A Laboratory Experiment. Insects 2020, 11, 808. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110808
Marini G, Manica M, Arnoldi D, Inama E, Rosà R, Rizzoli A. Influence of Temperature on the Life-Cycle Dynamics of Aedes albopictus Population Established at Temperate Latitudes: A Laboratory Experiment. Insects. 2020; 11(11):808. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110808
Chicago/Turabian StyleMarini, Giovanni, Mattia Manica, Daniele Arnoldi, Enrico Inama, Roberto Rosà, and Annapaola Rizzoli. 2020. "Influence of Temperature on the Life-Cycle Dynamics of Aedes albopictus Population Established at Temperate Latitudes: A Laboratory Experiment" Insects 11, no. 11: 808. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11110808