Ecological Niche Modeling to Calculate Ideal Sites to Introduce a Natural Enemy: The Case of Apanteles opuntiarum (Hymenoptera: Braconidae) to Control Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Distributional Data in Native Range
2.2. Model Calibration
2.3. Evaluation and Construction of Final Models
2.4. Extrapolation Risk Analysis and Selection of the Final Model
3. Results
3.1. Environmental Suitability of Cactoblastis cactorum in North America
3.2. Environmental Suitability for Apanteles opuntiarum in North America
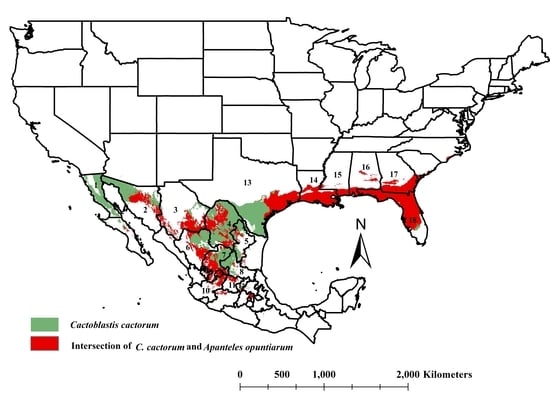
3.3. The Intersection of Cactoblastis cactorum and Apanteles opuntiarum Ecological Niches
3.4. Mess Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- López, C.J.; Malpica, A.; López, J.; García, E.; Sol, A. Crecimiento de Opuntia ficus-indica (L.) Mill. en la zona central de Veracruz. Rev. Mex. Cienc. Agríc. 2013, 5, 1005–1014. [Google Scholar]
- Anderson, E.F. The Cactus Family; Timber Press: Portland, OR, USA, 2001; p. 776. [Google Scholar]
- Vigueras, G.; Portillo, L. Uses of Opuntia species and the potential impact of Cactoblastis cactorum (Lepidoptera: Pyralidae) in Mexico. Fla. Entomol. 2001, 84, 493–498. [Google Scholar]
- Maki, G.; Peña, C.B.; García, R.; Arévalo, M.L.; Calderón, G.; Anaya, S. Características físicas y químicas de nopal verdura (Opuntia ficus-indica) para exportación y consumo nacional. Agrociencia 2015, 49, 31–51. [Google Scholar]
- Arba, M.; Falisse, A.; Choukr, R.; Sindic, M. Biology, flowering and fruiting of the cactus Opuntia spp.: A review and some observations on three varieties in Morocco. Braz. Arch. Biol. Technol. 2017, 60, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Márquez, S.R.; Torcuato, C.; Almaguer, G.; Colinas, M.T.; Gardez, A.K. El sistema productivo del nopal tunero (Opuntia albicarpa y O. megacantha) en Axapusco, Estado de México. Problemática y alternativas. Rev. Chapingo Ser. Hortic. 2012, 18, 81–93. [Google Scholar]
- Anuario Estadístico de la Producción Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 10 April 2020).
- Domínguez, I.A.; Granados, M.R.; Sagarnaga, L.M.; Salas, J.M.; Aguilar, J. Viabilidad económica y financiera de nopal tuna (Opuntia ficus-indica) en Nopaltepec, Estado de México. Rev. Mex. Cienc. Agríc. 2017, 8, 1371–1382. [Google Scholar]
- Vanegas, J.M.; Lomeli, J.R.; Rodríguez, E.; Mora, G.; Valdez, J.M. Enemigos naturales de Dactylopius opuntiae (Cockerell) en Opuntia ficus-indica (L.) Miller en el centro de México. Acta Zool. Mex. 2010, 26, 415–433. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, H.G.; Moran, V.C.; Hoffmann, J.H. The renowned cactus moth, Cactoblastis cactorum: Its natural history and threat to native Opuntia floras in Mexico and the United States of America. Divers. Distrib. 2000, 6, 259–269. [Google Scholar] [CrossRef]
- Zimmermann, H.G.; Pérez, M.; Goluvob, J.; Soberón, J.; Sarukhán, J. Cactoblastis cactorum, una nueva plaga de muy alto riesgo para las opuntias de México. Biodiversitas 2000, 33, 1–14. [Google Scholar]
- Gómez, G.C.; Neder, L.E.; Linares, M.A.; Zamar, M.I. Morfología de los estados inmaduros y biología de Cactoblastis Doddi (Lepidoptera: Pyralidae) en la prepuna de Jujuy (noroeste de Argentina). Rev. Biol. Trop. 2015, 63, 971–980. [Google Scholar] [CrossRef] [Green Version]
- Dodd, A.P. The Biological Campaign Against Prickly-Pear; Commonwealth Prickly Pear Board Bulletin: Brisbane, Australia, 1940; p. 177. [Google Scholar]
- Simmonds, F.J.; Bennett, F.D. Biological control of Opuntia spp. by Cactoblastis cactorum in the Leeward Islands (West Indies). Entomophaga 1966, 11, 183–189. [Google Scholar] [CrossRef]
- García-Turudi, J.C.; Martorell, L.F.; Medina Guad, S. Geographical distribution and host plant list of the cactus moth, Cactoblastis cactorum (Berg), in Puerto Rico and the United States Virgin Islands. J. Agric. Univ. Puerto Rico 1971, 55, 130–134. [Google Scholar]
- Dickel, T.S. Cactoblastis Cactorum in Florida (Lepidoptera: Pyralidae: Phycitinae). Trop. Lepid. 1991, 2, 117–118. [Google Scholar]
- Varone, L.; Goñalons, C.M.; Faltlhauser, A.C.; Guala, M.E.; Wolaver, D.; Srivastava, M.; Hight, S.D. Effect of rearing Cactoblastis cactorum on an artificial diet on the behavior of Apanteles opuntiarum. Appl. Entomol. 2020, 144, 278–286. [Google Scholar] [CrossRef]
- Hight, S.D.; Carpenter, J.E. Performance improvement through quality evaluations of sterile cactus moths, Cactoblastis cactorum (Lepidoptera: Pyralidae), mass-reared at two insectaries. Fla. Entomol. 2016, 99, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Hight, S.D.; Carpenter, J.E. Flight phenology of male Cactoblastis cactorum (Lepidoptera: Pyralidae) at different latitudes in the Southeastern United States. Fla. Entomol. 2009, 92, 208–216. [Google Scholar] [CrossRef]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a sterile insect release program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective overflooding ratios and release-recapture field studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.R.; Teal, P.E.A.; Epsky, N.D.; Dueben, B.D.; Hight, S.D.; Bloem, S.; Carpenter, J.E.; Weissling, T.J.; Kendra, P.E.; Cibrian-Tovar, J.; et al. Pheromone-based attractant for males of Cactobalstis cactorum (Lepidoptera: Pyralidae). Environ. Entomol. 2006, 35, 1469–1476. [Google Scholar] [CrossRef]
- Bloem, S.; Mizel, R.F., III; Bloem, K.A.; Hight, S.D.; Carpenter, J.E. Laboratory evaluation of insecticides for control of the invasive Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2005, 88, 395–400. [Google Scholar] [CrossRef]
- Pemberton, R.W.; Cordo, H.A. Potential and risks of biological control of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 2001, 84, 513–526. [Google Scholar] [CrossRef]
- Stiling, P. Potential non-target effects of a biological control agent, prickly pear moth, Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae) in North America, and possible management actions. Biol. Invasions 2002, 4, 273–281. [Google Scholar] [CrossRef]
- Martinez, J.J.; Berta, C.; Varone, L.; Logarzo, G.; Zamudio, P.; Zaldivar, A.; Aguilar, G. DNA barcoding and morphological identification of Argentine species of Apanteles (Hymenoptera: Braconidae), parasitoids of cactus-feeding moths (Lepidoptera: Pyralidae: Phycitinae), with description of a new species. Invertebr. Syst. 2012, 26, 435–444. [Google Scholar]
- Varone, L.; Logarzo, G.; Martínez, J.J.; Navarro, F.; Carpenter, J.E.; Hight, S.D. Field host range of Apanteles opuntiarum (Hymenoptera: Braconidae) in Argentina, a potential biocontrol agent of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 2015, 98, 803–806. [Google Scholar] [CrossRef]
- Mengoni, C.; Varone, L.; Logarzo, G.A.; Guala, M.; Rodriguero, M.S.; Hight, S.D.; Carpenter, J.E. Geographical range and laboratory studies on Apanteles opuntiarum (Hymenoptera: Braconidae) in Argentina, a candidate for biological control of Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Fla. Entomol. 2014, 97, 1458–1468. [Google Scholar]
- Srivastava, M.; Srivastava, P.; Karan, R.; Jeyaprakash, A.; Whilby, L.; Rohrig, E.; Howe, A.C.; Hight, S.D.; Varone, L. Molecular detection method developed to track the koinobiont larval parasitoid Apanteles opuntiarum (Hymenoptera: Braconidae) imported from Argentina to control Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2019, 102, 329–335. [Google Scholar]
- Mills, N.J. An alternative perspective for the theory of biological control. Insects 2018, 9, 131. [Google Scholar]
- Schulz, A.N.; Lucardi, R.D.; Marsico, T.D. Successful invasions and failed biocontrol: The role of antagonistic species interactions. BioScience 2019, 69, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Olfert, O.; Haye, T.; Weiss, R.; Kriticos, D.; Kuhlmann, U. Modelling the potential impact of climate change on future spatial and temporal patterns of biological control agents: Peristenus digoneutis (Hymenoptera: Braconidae) as a case study. Can. Entomol. 2016, 148, 579–594. [Google Scholar] [CrossRef]
- Robertson, M.P.; Kriticos, D.J.; Zachariades, C. Climate matching techniques to narrow the search for biological control agents. Biol. Control 2008, 46, 442–452. [Google Scholar]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar]
- Suárez-Mota, M.E.; Ortiz, E.; Villaseñor, J.L.; Espinosa-García, F.J. Ecological niche modeling of invasive plant species according to invasion status and management needs: The case of Chromolaena odorata (Asteraceae) in South Africa. Pol. J. Ecol. 2016, 64, 369–383. [Google Scholar] [CrossRef]
- Kantola, T.; Tracy, J.L.; Lyytikäinen-Saarenmaa, P.; Saarenmaa, H.; Coulson, R.N.; Trabucco, A.; Holopainen, M. Hemlock woolly adelgid niche models from the invasive eastern North American range with projections to native ranges and future climates. iForest 2019, 12, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Hyseni, C.; Garrick, R.C. Ecological drivers of species distributions and niche overlap for three subterranean termite species in the Southern Appalachian Mountains, USA. Insects 2019, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Oxfordshire, UK, 2011; p. 314. [Google Scholar]
- Owens, H.L.; Campbell, L.P.; Dornak, L.; Saupe, E.E.; Barve, N.; soberón, J.; Ingenloff, K.; Lira-Noriega, A.; Hensz, C.M.; Myers, C.E.; et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.D.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologist. Divers. Distrib. 2010, 1, 1–15. [Google Scholar] [CrossRef]
- Melo, S.M.; Reyes, H.; Lira, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 1–35. [Google Scholar]
- Sweets, K.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.T.; Papes, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Qiao, H.; Feng, X.; Escobar, L.E.; Peterson, A.T.; Soberón, J.; Zhu, G.; Papes, M. An evaluation of transferability of ecological niche models. Ecography 2019, 42, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Galante, P.J.; Alade, B.; Muscarella, R.; Jansa, S.A.; Goodman, S.M.; Anderson, R.P. The challenge of modeling niches and distributions for data-poor species: A comprehensive approach to model complexity. Ecography 2018, 41, 726–736. [Google Scholar] [CrossRef] [Green Version]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Elith, J.; Kearny, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Broennimann, O.; Mráz, P.; Petitpierre, B.; Guisan, A.; Müller-Schärer, H. Contrasting spatio-temporal climatic niche dynamics during the eastern and western invasions of spotted knapweed in North America. J. Biogeogr. 2014, 41, 1126–1136. [Google Scholar] [CrossRef] [Green Version]
- Mandle, L.; Warren, D.L.; Hoffman, M.H.; Peterson, A.T.; Schmitt, J.; von Wettberg, E.J. Conclusions about niche expansion in introduced Impatiens walleriana populations depend on method of analysis. PLoS ONE 2010, 5, e15297. [Google Scholar] [CrossRef] [Green Version]
- Wakie, T.T.; Neven, L.G.; Yee, W.L.; Lu, Z. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 2020, 113, 306–314. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States; U.S. Geological Survey: Reston, VA, USA, 2012; p. 10.
- Simonsen, T.J.; Brown, R.L.; Sperling, F.A.H. Tracing an invasion: Phylogeography of Cactoblastis cactorum (Lepidoptera: Pyralidae) in the United States based on mitochondrial DNA. Ann. Entomol. Soc. Am. 2008, 101, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Marsico, T.D.; Wallace, L.E.; Ervin, G.N.; Brooks, C.P.; McClure, J.E.; Welch, M.E. Geographic patterns of genetic diversity from the native range of Cactoblastis cactorum (Berg) support the documented history of invasion and multiple introductions for invasive populations. Biol. Invasions 2011, 13, 857–868. [Google Scholar] [CrossRef]
- Andraca-Gómez, G.; Ordano, M.; Boege, K.; Domínguez, C.A.; Piñero, D.; Pérez-Ishiwara, R.; Pérez-Camacho, J.; Cañizares, M.; Fornoni, J. A potential invasion route of Cactoblastis cactorum within the Caribbean region matches historical hurricane trajectories. Biol. Invasions 2015, 17, 1397–1406. [Google Scholar] [CrossRef]
- Schartel, T.; Brooks, C. Biotic constraints on Cactoblastis cactorum (Berg) host use in the southern US and their implications for future spread. Food Webs 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Jezorek, H.; Baker, A.J.; Stiling, P. Effects of Cactoblastis cactorum on the survival and growth of North American Opuntia. Biol. Invasions 2012, 14, 2355–2367. [Google Scholar] [CrossRef]
- Johnson, D.M.; Stiling, P.D. Host specificity of Cactoblastis cactorum (Lepidoptera: Pyralidae), an exotic Opuntia-feeding moth, in Florida. Environ. Entomol. 1996, 25, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Morrone, J.J. Hacia una síntesis biogeográfica de México. Rev. Mex. Biodiv. 2005, 76, 207–252. [Google Scholar] [CrossRef]
- Bravo-Hollis, H.H.; Sánchez-Mejorada, H. Las cactáceas de México; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1978; Volume 1, p. 743. [Google Scholar]
- Majure, L.C.; Puente, R.; Griffith, M.P.; Judd, W.S.; Soltis, P.S.; Soltis, D.E. Phylogeny of Opuntia s.s. (Cactaceae): Clade delineation, geographic origin, and reticulate evolution. Am. J. Bot. 2012, 99, 847–864. [Google Scholar] [CrossRef] [Green Version]
- Galicia, S.; Escamilla, P.E.; Alvarado, H.; Aquino, L.V.; Serna, H.; Hernández, L.M. Plantación experimental de nopal para evaluación de sistemas de fertilización y extracción de mucílago. Rev. Mex. Cienc. Agríc. 2017, 8, 1097–1099. [Google Scholar]
- Badii, M.H.; Flores, A.E. Prickly pear cacti pests and their control in Mexico. Fla. Entomol. 2001, 84, 503–505. [Google Scholar] [CrossRef]
- Flores, J.L.; Mora, G.; Loeza, E.; López, J.I.; Domínguez, S.; Acevedo, G.; Robles, P. Pérdidas en producción inducidas por Candidatus Liberibacter asiaticus en limón Persa, en Yucatán, México. Rev. Fito. Méx. 2015, 3, 195–210. [Google Scholar]
- Rosas, N.M.; Parra, G.M. Incidencia de la cochinilla rosada del hibisco en cultivares de mango de Nayarit, México. Acta Zool. Mex. 2011, 27, 407–418. [Google Scholar]
- López-Collado, J.; López-Arroyo, J.I.; Robles-García, P.L.; Márquez-Santos, M. Geographic distribution of habitat, development, and population growth rates of the Asian citrus psyllid, Diaphorina citri, in Mexico. J. Insect Sci. 2013, 13, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isiordia-Aquino, N.; Robles-Bermúdez, A.; García-Martínez, O.; Lomelí-Flores, R.; Flores-Canales, R.; Gómez-Aguilar, J.R.; Espino-Alvarez, R. Especies forestales y arbustivas asociadas a Maconellicoccus hirsutus (Green) (Hemiptera: Pseudococcidae) en el norte de Nayarit, México. Acta Zool. Mex. 2012, 28, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive plants distribution modeling: A tool for tropical biodiversity conservation with special reference to Sri Lanka. Trop. Conserv. Sci. 2019, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Trethowan, P.D.; Robertson, M.P.; McConnachie, A.J. Ecological niche modelling of an invasive alien plant and its potential biological control agents. S. Afr. J. Bot. 2011, 77, 137–146. [Google Scholar] [CrossRef] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-De la O, N.B.; Espinosa-Zaragoza, S.; López-Martínez, V.; D. Hight, S.; Varone, L. Ecological Niche Modeling to Calculate Ideal Sites to Introduce a Natural Enemy: The Case of Apanteles opuntiarum (Hymenoptera: Braconidae) to Control Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Insects 2020, 11, 454. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11070454
Pérez-De la O NB, Espinosa-Zaragoza S, López-Martínez V, D. Hight S, Varone L. Ecological Niche Modeling to Calculate Ideal Sites to Introduce a Natural Enemy: The Case of Apanteles opuntiarum (Hymenoptera: Braconidae) to Control Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America. Insects. 2020; 11(7):454. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11070454
Chicago/Turabian StylePérez-De la O, Nidia Bélgica, Saúl Espinosa-Zaragoza, Víctor López-Martínez, Stephen D. Hight, and Laura Varone. 2020. "Ecological Niche Modeling to Calculate Ideal Sites to Introduce a Natural Enemy: The Case of Apanteles opuntiarum (Hymenoptera: Braconidae) to Control Cactoblastis cactorum (Lepidoptera: Pyralidae) in North America" Insects 11, no. 7: 454. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11070454