Effect of Dinotefuran, Permethrin, and Pyriproxyfen (Vectra® 3D) on the Foraging and Blood-Feeding Behaviors of Aedes albopictus Using Laboratory Rodent Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals and Ethics Statement
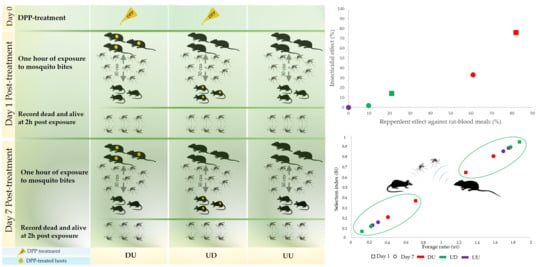
2.2. Treatment Administration
2.3. Source of Ae. albopictus
2.4. Animal Exposure to Ae. albopictus
2.5. Engorgement Determination and Blood-Meal Identification
2.6. Data Analysis
3. Results
3.1. Insecticidal Efficacy
3.2. Direct and Indirect Anti-Feeding Efficacy
3.3. Host Preference
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Material
Ethics Approval and Consent to Participate
References
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. Aedes albopictus—current known distribution. Available online: https://www.ecdc.europa.eu/en/publications-data/aedes-albopictus-current-known-distribution-may-2020 (accessed on 15 May 2015).
- Reiter, P.; Sprenger, D. The used tire trade: A mechanism for the worldwide dispersal of container breeding mosquitoes. J. Am. Mosq. Control Assoc. 1987, 3, 494. [Google Scholar] [PubMed]
- World Health Organisation (WHO). WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care; World Health Organisation (WHO): Geneva, Switzerland, 2009. [Google Scholar]
- Abramides, G.C.; Roiz, D.; Guitart, R.; Quintana, S.; Guerrero, I.; Giménez, N. Effectiveness of a multiple intervention strategy for the control of the tiger mosquito (Aedes albopictus) in Spain. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worobey, J.; Fonseca, D.M.; Espinosa, C.; Healy, S.; Gaugler, R. Child outdoor physical activity is reduced by prevalence of the Asian tiger mosquito, Aedes albopictus. J. Am. Mosq. Control Assoc. 2013, 29, 78–80. [Google Scholar] [CrossRef]
- Eritja, R.; Escosa, R.; Lucientes, J.; Marquès, E.; Molina, R.; Roiz, D.; Ruiz, S. Worldwide invasion of vector mosquitoes: Present European distribution and challenges for Spain. Biol. Invasions 2005, 7, 87–97. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Gasperi, G.; Anthony, A.J. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol. 2014, 29, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.L.; Ponnusamy, L.; Unnasch, T.R.; Hassan, H.K.; Apperson, C.S. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J. Med. Entomol. 2006, 43, 543–551. [Google Scholar] [CrossRef]
- Pereira-Dos-Santos, T.; Roiz, D.; Lourenço-De-Oliveira, R.; Paupy, C. A systematic review: Is Aedes albopictus an efficient bridge vector for zoonotic arboviruses? Pathogens 2020, 9, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Paupy, C.; Delatte, H.; Bagny, L.; Corbel, V.; Fontenille, D. Aedes albopictus, an arbovirus vector: From the darkness to the light. Microbes Infect. 2009, 11, 1177–1185. [Google Scholar] [CrossRef]
- Simón, F.; Siles-lucas, M.; Morchón, R.; González-miguel, J.; Mellado, I.; Carretón, E.; Montoya-alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [Green Version]
- Dantas-Torres, F.; Otranto, D. Best practices for preventing vector-borne diseases in dogs and humans. Trends Parasitol. 2016, 32, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mccall, J.W.; Varloud, M.; Hodgkins, E.; Mansour, A.; Dicosty, U.; Mccall, S.; Carmichael, J.; Carson, B.; Carter, J. Shifting the paradigm in Dirofilaria immitis prevention: Blocking transmission from mosquitoes to dogs using repellents/insecticides and macrocyclic lactone prevention as part of a multimodal approach. Parasites Vectors 2017, 10, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, J.W.; Hodgkins, E.; Varloud, M.; Mansour, A.; Dicosty, U. Blocking the transmission of heartworm (Dirofilaria immitis) to mosquitoes (Aedes aegypti) by weekly exposure for one month to microfilaremic dogs treated once topically with dinotefuran-permethrin-pyriproxyfen. Parasites Vectors 2017, 10, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahir, D.; Davoust, B.; Almeras, L.; Berenger, J.M.; Varloud, M.; Parola, P. Anti-feeding and insecticidal efficacy of a topical administration of dinotefuran–pyriproxyfen–permethrin spot-on (Vectra® 3D) on mice against Stegomyia albopicta (= Aedes albopictus). Med. Vet. Entomol. 2017, 31, 351–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartereau, A.; Houchat, J.N.; Mannai, S.; Varloud, M.; Karembé, H.; Graton, J.; Le Questel, J.Y.; Thany, S.H. Permethrin enhances the agonist activity of dinotefuran on insect cholinergic synaptic transmission and isolated neurons. Neurotoxicology 2018, 67, 206–214. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M. Atypical Topical Spot-On Products, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 2013; pp. 741–754. ISBN 9781455707171. [Google Scholar]
- Agency, E.M. Guideline on data requirements for veterinary medicinal products for the prevention of transmission of vector- borne diseases in dogs and cats. Eur. Med. Agency 2018, 44, 1–11. [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration. E 10 Choice of Control Group and Related Issues in Clinical Trials; U.S. Department of Health and Human Services Food And Drug Administration: Maryland, DC, USA, 2009; Volume 2012.
- Legifrance Decree No. 2013–118 of 1 February 2013 Concerning the Protection of Animals Used for Scientific Purposes. 2013. Available online: https://www.legifrance.gouv.fr/eli/decret/2013/2/1/AGRG1231951D/jo/texte (accessed on 15 August 2015).
- Freireich, E.J.; Gehan, E.A.; Rall, D.P.; Schmidt, L.H.; Skipper, H.E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966, 50, 219. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Blanga-Kanfi, S.; Miranda, H.; Penn, O.; Pupko, T.; Debry, R.W.; Huchon, D. Rodent phylogeny revised: Analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 2009, 9, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Céspedes, H.A.; Zavala, K.; Vandewege, M.W.; Opazo, J.C. Evolution of the α2-adrenoreceptors in vertebrates: ADRA2D is absent in mammals and crocodiles. Gen. Comp. Endocrinol. 2017, 250, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Douzery, E.J.P.; Delsuc, F.; Stanhope, M.J.; Huchon, D. Local molecular clocks in three nuclear genes: Divergence times for rodents and other mammals and incompatibility among fossil calibrations. J. Mol. Evol. 2003, 57, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, R.A.; Weinstock, G.M.; Metzker, M.L.; Muzny, D.M.; Sodergren, E.J.; Scherer, S.; Scott, G.; Steffen, D.; Worley, K.C.; Burch, P.E.; et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 2004, 428, 493–520. [Google Scholar] [PubMed] [Green Version]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Younes, L.; Bernard, D.; Marie, V.; Niang, E.H.A.; Fenollar, F.; Mediannikov, O. Development of a multiplexed qPCRs-based approach for the diagnosis of Dirofilaria immitis, D. repens, Acanthocheilonema reconditum and the others filariosis. BioRxiv 2019, 24, 5–27. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar]
- Bashar, K.; Tuno, N.; Ahmed, T.; Howlader, A. Blood-feeding patterns of Anopheles mosquitoes in a malaria-endemic area of Bangladesh. Parasites Vectors 2012, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Silver, J.B. Mosquito Ecology-Field Sampling Methods, 3rd ed.; Springer science & business media: Berlin, Germany, 2008; ISBN 978-1-4020-6666-5. [Google Scholar]
- Hess, A.D.; Richard, O.; Hayes, C.H.T. The use of the forage ratio technique in mosquito host preference studies. Mosq. News 1968, 23, 386–388. [Google Scholar]
- Manly, B.F.L.; McDonald, L.; Thomas, D.L.; McDonald, T.L.; Erickson, W.P. Resource Selection by Animals. Statistical Design and Analysis for Field Studies; Springer: London, UK, 1993; Volume 53, ISBN 9781402006777. [Google Scholar]
- World Health Organization. WHO Pesticide Evaluation Scheme Guidelines for Efficacy Testing of Spatial Repellents; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets (No. Who/Cds/Ntd/Whopes/Gcdpp/2006.3); World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Franc, M.; Genchi, C.; Bouhsira, E.; Warin, S.; Kaltsatos, V.; Baduel, L.; Genchi, M. Efficacy of dinotefuran, permethrin and pyriproxyfen combination spot-on against Aedes aegypti mosquitoes on dogs. Vet. Parasitol. 2012, 189, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Bouhsira, E.; Liénard, E.; Lyazrhi, F.; Jacquiet, P.; Varloud, M.; Deflandre, A.; Franc, M. Repellent and insecticidal efficacy of a combination of dinotefuran, pyriproxyfen and permethrin (Vectra® 3D) against Culex pipiens in dogs. Parasite Epidemiol. Control 2016, 1, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delatte, H.; Desvars, A.; Bouétard, A.; Bord, S.; Gimonneau, G.; Vourc’h, G.; Fontenille, D. Blood-feeding behavior of Aedes albopictus, a vector of chikungunya on la réunion. Vector-Borne Zoonotic Dis. 2010, 10, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogoma, S.B.; Moore, S.J.; Maia, M.F. A systematic review of mosquito coils and passive emanators: Defining recommendations for spatial repellency testing methodologies. Parasites Vectors 2012, 5, 287. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.; Rowland, M. Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Trop. Med. Int. Heal. 1999, 4, 481–486. [Google Scholar] [CrossRef] [Green Version]
- Habtewold, T.; Prior, A.; Torr, S.J.; Gibson, G. Could insecticide-treated cattle reduce Afrotropical malaria transmission? Effects of deltamethrin-treated Zebu on Anopheles arabiensis behaviour and survival in Ethiopia. Med. Vet. Entomol. 2004, 18, 408–417. [Google Scholar] [CrossRef]
- Kawada, H.; Ohashi, K.; Dida, G.O.; Sonye, G.; Njenga, S.M.; Mwandawiro, C.; Minakawa, N. Insecticidal and repellent activities of pyrethroids to the three major pyrethroid-resistant malaria vectors in western Kenya. Parasites Vectors 2014, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Koama, B.; Namountougou, M.; Sanou, R.; Ndo, S.; Ouattara, A.; Dabiré, R.K.; Malone, D.; Diabaté, A. The sterilizing effect of pyriproxyfen on the malaria vector Anopheles gambiae: Physiological impact on ovaries development. Malar. J. 2015, 14, 101. [Google Scholar] [CrossRef] [Green Version]
- Grieco, J.P.; Achee, N.L.; Chareonviriyaphap, T.; Suwonkerd, W.; Chauhan, K.; Sardelis, M.R.; Roberts, D.R. A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS ONE 2007, 2, e716. [Google Scholar] [CrossRef] [Green Version]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative host feeding patterns of the asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis. 2014, 8, e3037. [Google Scholar] [CrossRef] [Green Version]


| Species Description | Active Product | Conversion Factor from Dogs (dog dose mg/kg BW) | Dose mg/kg BW | Correction Factor (km) | Dose mg/m2 BSA | Dose Per Animal, mg | Vectra® 3D (mL/animal unit) |
|---|---|---|---|---|---|---|---|
| Mouse (0.02 kg BW, 0.0066 m2 BSA) | Dinotefuran | 6 (6.4) | 38.4 | 3 | 115.2 | 0.76 | 0.014 |
| Pyriproxyfen | 6 (0.6) | 3.6 | 3 | 10.8 | 0.071 | ||
| Permethrin | 6 (46.6) | 279.6 | 3 | 838.8 | 5.54 | ||
| Rat (0.15 kg BW, 0.025 m2 BSA) | Dinotefuran | 4 (6.4) | 25.6 | 6 | 153.6 | 3.84 | 0.070 |
| Pyriproxyfen | 4 (0.6) | 2.4 | 6 | 14.4 | 0.36 | ||
| Permethrin | 4 (46.6) | 186.4 | 6 | 1118.4 | 27.96 |
| Molecular Identification of Blood-Meals | Viability at 2 h after Exposure | Total Used Per Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Statistics | Feed on Rats | Feed on Mice | Feed on Both | Engorged | Dead | Alive | ||
| Day 1 | DU | 15 (4.9) | 8 (2.3) | 1 (0.6) | 24 (7.9) | 162 (53.6) | 53 (16.1) | 215 (71.6) |
| UD | 74 (21.9) | 2 (0.6) | 3 (0.8) | 79 (23.0) | 41 (13.5) | 172 (57.3) | 213 (71.0) | |
| UU | 85 (28.2) | 10 (3.2) | 2 (0.6) | 97 (32.1) | 8 (2.4) | 201 (66.9) | 209 (96.5) | |
| Kruskal Wallis test | ||||||||
| p-value | 0.049 | 0.997 | 0.996 | 0.043 | 0.004 | 0.004 | / | |
| Pairwise comparisons (p-value) | ||||||||
| DU vs. UD | 0.015 | 0.079 | 0.674 | 0.025 | 0.010 | 0.009 | / | |
| DU vs. UU | 0.027 | 0.430 | 1.000 | 0.014 | <0.0001 | <0.0001 | / | |
| UD vs. UU | 0.657 | 0.025 | 0.674 | 0.653 | 0.010 | 0.009 | / | |
| Day 7 | DU | 33 (10.9) | 7 (2.1) | 2 (0.6) | 42 (13.7) | 96 (31.3) | 129 (42.6) | 225 (75.0) |
| UD | 78 (26.0) | 8 (2.4) | 2 (0.6) | 88 (29.3) | 31 (10.0) | 187 (62.3) | 218 (72.6) | |
| UU | 87 (28.6) | 13 (3.9) | 3 (0.8) | 103 (33.6) | 13 (4.2) | 191 (63.6) | 204 (68.0) | |
| Kruskal Wallis test | ||||||||
| p-value | 0.039 | 0.561 | 0.996 | 0.05 | 0.004 | 0.029 | / | |
| Pairwise comparisons (p-value) | ||||||||
| DU vs. UD | 0.028 | 0.833 | 1.000 | 0.030 | 0.010 | 0.028 | / | |
| DU vs. UU | 0.012 | 0.314 | 0.674 | 0.012 | <0.0001 | 0.012 | / | |
| UD vs. UU | 0.500 | 0.413 | 0.674 | 0.506 | 0.010 | 0.50 | / | |
| Groups/Times | Mortality Statutes of Ae. albopictus | Insecticidal Effect (%) | ||
|---|---|---|---|---|
| GM | Percentage | |||
| Day 1 | DU | 53.6 | 74.9 | 75.9 |
| UD | 13.5 | 19.1 | 14.3 | |
| UU | 2.4 | 3.5 | / | |
| Day 7 | DU | 31.3 | 41.7 | 33.0 |
| UD | 10.0 | 13.8 | 2.0 | |
| UU | 4.2 | 6.2 | / | |
| Groups/Times | Engorged Ae. albopictus | Percent of Blood-Meals (% Ri) | Repellent Effect (%) | |||
|---|---|---|---|---|---|---|
| GM | Percentage | Rats | Mice | Rat | ||
| Day 1 | DU | 7.9 | 11.2 | 7.4 | 4.2 | 81.9 a |
| UD | 23.0 | 37.1 | 35.7 | 2.3 | 21.1 b | |
| UU | 32.1 | 46.4 | 41.2 | 5.7 | / | |
| Day 7 | DU | 13.7 | 18.7 | 15.4 | 4.0 | 61.0 a |
| UD | 29.3 | 40.4 | 36.4 | 4.6 | 9.9 b | |
| UU | 33.6 | 50.5 | 43.5 | 7.7 | / | |
| Groups | Percent of Blood-Meals (% Oi) | Forage Ratio for Host i (wi) | Selection Index for Host i (Bi) | ||||
|---|---|---|---|---|---|---|---|
| Rat | Mouse | Rat | Mouse | Rat | Mouse | ||
| Day 1 | DU | 64.0 | 36.0 | 1.28 | 0.72 | 0.64 | 0.36 |
| UD | 93.9 | 6.1 | 1.88 | 0.12 | 0.94 | 0.06 | |
| UU | 87.9 | 12.1 | 1.76 | 0.24 | 0.88 | 0.12 | |
| Day 7 | DU | 79.6 | 20.4 | 1.59 | 0.41 | 0.80 | 0.20 |
| UD | 88.9 | 11.1 | 1.78 | 0.22 | 0.89 | 0.11 | |
| UU | 84.9 | 15.1 | 1.70 | 0.30 | 0.85 | 0.15 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laidoudi, Y.; Tahir, D.; Medkour, H.; Varloud, M.; Mediannikov, O.; Davoust, B. Effect of Dinotefuran, Permethrin, and Pyriproxyfen (Vectra® 3D) on the Foraging and Blood-Feeding Behaviors of Aedes albopictus Using Laboratory Rodent Model. Insects 2020, 11, 507. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11080507
Laidoudi Y, Tahir D, Medkour H, Varloud M, Mediannikov O, Davoust B. Effect of Dinotefuran, Permethrin, and Pyriproxyfen (Vectra® 3D) on the Foraging and Blood-Feeding Behaviors of Aedes albopictus Using Laboratory Rodent Model. Insects. 2020; 11(8):507. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11080507
Chicago/Turabian StyleLaidoudi, Younes, Djamel Tahir, Hacène Medkour, Marie Varloud, Oleg Mediannikov, and Bernard Davoust. 2020. "Effect of Dinotefuran, Permethrin, and Pyriproxyfen (Vectra® 3D) on the Foraging and Blood-Feeding Behaviors of Aedes albopictus Using Laboratory Rodent Model" Insects 11, no. 8: 507. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11080507