Structure of the Assemblages of Spiders in Mediterranean Pear Orchards and the Effect of Intensity of Spraying
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location, Setting, and Management of the Orchards
2.2. Sampling of Spiders
2.3. Data Analyses
2.3.1. Analysis of the Richness of Genera
2.3.2. Analysis of the Abundance and Population Dynamics of Spiders
2.3.3. Analysis of the Structure of Spider Assemblages
3. Results
3.1. Samples, Composition, and Richness of the Spider Assemblage
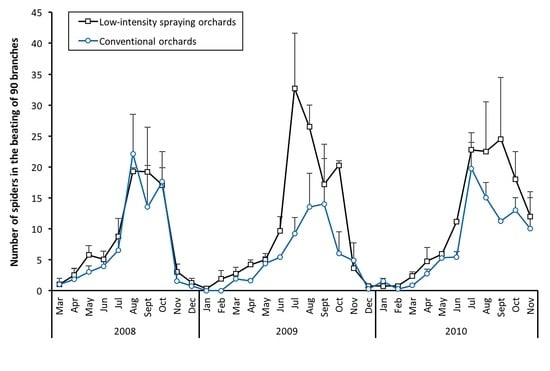
3.2. Population Dynamics of Spiders
3.3. Abundance and Trend of Families/Guilds
3.4. Structure of the Assemblage of Spiders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nyffeler, M.; Birkhofer, K. An estimated 400–800 million tons of prey are annually killed by the global spider community. Sci. Nat. 2017, 104, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platnick, N.I. World Spider Catalog. Version 21.0. Available online: http://wsc.nmbe.ch (accessed on 24 March 2020).
- Bogya, S.; Markó, V.; Szinetár, C. Comparison of pome fruit orchard inhabiting spider assemblages at different geographical scales. Agric. For. Entomol. 1999, 1, 261–269. [Google Scholar] [CrossRef]
- Bogya, S.; Markó, V.; Szinetár, C. Effect of pest management systems on foliage- and grass-dwelling spider communities in an apple orchard in Hungary. Int. J. Pest Manag. 2000, 46, 241–250. [Google Scholar] [CrossRef]
- Horton, D.R.; Broers, D.A.; Lewis, R.R.; Granatstein, D.; Zack, R.S.; Unruh, T.R.; Moldenke, A.R.; Brown, J.J. Effects of mowing frequency on densities of natural enemies in three Pacific Northwest pear orchards. Entomol. Exp. Appl. 2003, 106, 135–145. [Google Scholar] [CrossRef]
- DeBach, P.; Rosen, D. Biological Control by Natural Enemies; Cambridge University Press: New York, NY, USA, 1991. [Google Scholar]
- Solomon, M.G.; Cross, J.V.; Fitzgerald, J.D.; Campbell, C.A.M.; Jolly, R.L.; Olszak, R.W.; Niemczyk, E.; Vogt, H. Biocontrol of pests of apples and pears in northern and central Europe-3. Predators. Biocontrol Sci. Technol. 2000, 10, 91–128. [Google Scholar] [CrossRef]
- Mansour, F.; Rosen, D.; Shulov, A. A survey of spider populations (Araneae) in sprayed and unsprayed apple orchards in Israel and their ability to feed on larvae of Spodoptera littoralis (Boisd.). Acta Oecol. Oecol. Appl. 1980, 1, 189–197. [Google Scholar]
- Van der Blom, J.; Drukker, B.; Blommers, L. The possible significance of various groups of predators in preventing pear Psylla outbreaks. Meded. Fac. Landbouwwet. Rijksuniv. Gent 1985, 50, 419–424. [Google Scholar]
- Wyss, E.; Niggli, U.; Nentwig, W. The impact of spiders on aphid populations in a strip-managed apple orchard. J. Appl. Entomol. 1995, 119, 473–478. [Google Scholar] [CrossRef]
- Wisniewska, J.; Prokopy, R.J. Do spiders (Araneae) feed on rose leafhopper (Edwardsiana rosae; Auchenorrhyncha: Cicadellidae) pests of apple trees? Eur. J. Entomol. 1997, 94, 243–251. [Google Scholar]
- Marc, P.; Canard, A.; Ysnel, F. Spiders (Araneae) useful for pest limitation and bioindication. Agric. Ecosyst. Environ. 1999, 74, 229–273. [Google Scholar] [CrossRef]
- Nyffeler, M.; Sunderland, K.D. Composition, abundance and pest control potential of spider communities in agroecosystems: A comparison of European and US studies. Agric. Ecosyst. Environ. 2003, 95, 579–612. [Google Scholar] [CrossRef]
- Riechert, S.E.; Lockley, T. Spiders as biological control agents. Annu. Rev. Entomol. 1984, 29, 299–320. [Google Scholar] [CrossRef]
- Eurostat (Statistical Office of the European Union) Database. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 20 January 2020).
- MAPA (Ministerio de Agricultura, Pesca y Alimentación). Anuario de Estadística. Available online: https://www.mapa.gob.es/es/estadistica/temas/default.aspx (accessed on 20 January 2020).
- Rieux, R.; Lyoussoufi, A.; Armand, E.; D’Arcier, F.F. Dynamics of winter and post-winter populations of the pear psylla Psylla pyri (L.) (Homoptera: Psyllidae). Acta Phytopathol. Entomol. Hung. 1992, 27, 545–549. [Google Scholar]
- Vilajeliu, M.; Lloret, P.; Vilardell, P. Dinámica poblacional de la psila (Cacopsylla pyri L.) y de sus enemigos naturales en plantaciones comerciales de peral de Girona. Boletín Sanid. Veg. Plagas 1998, 24, 231–238. [Google Scholar]
- Stamenkovic, S.; Milenkovic, S.; Injac, M. Population numbers, harmfulness and control of pear psylla (Cacopsylla pyri L.) in Serbia. IOBC WPRS Bull. 2001, 24, 145–150. [Google Scholar]
- Erler, F. Natural enemies of the pear psylla Cacopsylla pyri in treated vs untreated pear orchards in Antalya, Turkey. Phytoparasitica 2004, 32, 295–304. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Ortín-Angulo, M.C. Abundance and population dynamics of Cacopsylla pyri (Hemiptera: Psyllidae) and its potential natural enemies in pear orchards in southern Spain. Crop Prot. 2012, 32, 24–29. [Google Scholar] [CrossRef]
- Civolani, S.; Pasqualini, E. Cacopsylla pyri L. (Hom., Psyllidae) and its predators relationship in Italy’s Emilia-Romagna region. J. Appl. Entomol. 2003, 127, 214–220. [Google Scholar] [CrossRef]
- Civolani, S.; Cassanelli, S.; Rivi, M.; Manicardi, G.C.; Peretto, R.; Chicca, M.; Pasqualini, E.; Leis, M. Survey of susceptibility to abamectin of pear psylla (Hemiptera: Psyllidae) in northern Italy. J. Econ. Entomol. 2010, 103, 816–822. [Google Scholar] [CrossRef]
- Ehler, L.E. Integrated pest management (IPM): Definition, historical development and implementation, and the other IPM. Pest Manag. Sci. 2006, 62, 787–789. [Google Scholar] [CrossRef]
- Sanchez, J.A.; López-Gallego, E.; La-Spina, M. The impact of ant mutualistic and antagonistic interactions on the population dynamics of sap-sucking hemipterans in pear orchards. Pest Manag. Sci. 2020, 76, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.A.; Carrasco-Ortiz, A.; López-Gallego, E.; La-Spina, M. Ants (Hymenoptera: Formicidae) reduce the density of Cacopsylla pyri (Linnaeus, 1761) in Mediterranean pear orchards. Myrmecol. News 2020, 30, 93–102. [Google Scholar]
- Chant, D.A. Predacious spiders in orchards in south-eastern England. J. Hortic. Sci. 1956, 31, 35–46. [Google Scholar] [CrossRef]
- Samu, F.; Lovei, G.L. Species richness of a spider community (Araneae): Extrapolation from simulated increasing sampling effort. Eur. J. Entomol. 1995, 92, 633–638. [Google Scholar]
- Samu, F.; Rácz, V.; Erdélyi, C.; Balázs, K. Spiders of the foliage and herbaceous layer of an IPM apple orchard in Kecskemét-Szarkás, Hungary. Biol. Agric. Hortic. 1997, 15, 131–140. [Google Scholar] [CrossRef]
- Pekár, S. Effect of selective insecticides on the beneficial spider community of a pear orchard in the Czech Republic. Proc. 17th Eur. Colloq. Arachnol. 1998, 27, 337–342. [Google Scholar]
- Bogya, S.; Szinetár, C.; Markó, V. Species composition of spider (Araneae) assemblages in apple and pear orchards in the Carpathian Basin. Acta Phytopathol. Entomol. Hung. 1999, 34, 99–121. [Google Scholar]
- Pekár, S. Foraging mode: A factor affecting the susceptibility of spiders (Araneae) to insecticide applications. Pestic. Sci. 1999, 55, 1077–1082. [Google Scholar] [CrossRef]
- Pekár, S.; Haddad, C.R. Can agrobiont spiders (Araneae) avoid a surface with pesticide residues? Pest Manag. Sci. 2005, 61, 1179–1185. [Google Scholar] [CrossRef]
- Markó, V.; Keresztes, B.; Fountain, M.T.; Cross, J.V. Prey availability, pesticides and the abundance of orchard spider communities. Biol. Control 2009, 48, 115–124. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Dondale, C.D.; Binns, M.R.; Pitre, D. Effects of pesticide use on spiders (Araneae) in Quebec apple orchards. Can. Entomol. 1984, 116, 663–675. [Google Scholar] [CrossRef]
- Miliczky, E.R.; Calkins, C.O.; Horton, D.R. Spider abundance and diversity in apple orchards under three insect pest management programmes in Washington State, U.S.A. Agric. For. Entomol. 2000, 2, 203–215. [Google Scholar] [CrossRef]
- Khan, A.A. Comparison of spider diversity in relation to pesticide use in apple orchards of Kashmir. J. Biol. Control 2012, 26, 1–10. [Google Scholar]
- Mazzia, C.; Pasquet, A.; Caro, G.; Thénard, J.; Cornic, J.F.; Hedde, M.; Capowiez, Y. The impact of management strategies in apple orchards on the structural and functional diversity of epigeal spiders. Ecotoxicology 2015, 24, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Mazzia, C.; Capowiez, Y.; Marliac, G.; Josselin, D.; Pasquet, A. Spinosad application in an apple orchard affects both the abundance of the spider Araneus diadematus and its web construction behaviour. Ecotoxicology 2020, 29, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Olszak, R.W.; Luczak, L.; Niemczyk, E.; Zajac, R.Z. The spider community associated with apple trees under different pressure of pesticides. Ekol. Pol. 1992, 40, 265–286. [Google Scholar]
- R-Development-Core-Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Statistic and Computing. MODERN Applied Statistic with S.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Uetz, G.W.; Halaj, J.; Cady, A.B. Guild structure of spiders in major crops. J. Arachnol. 1999, 27, 270–280. [Google Scholar]
- Nyffeler, M.; Benz, G. Spiders in natural pest control: A review. J. Appl. Entomol. 1987, 12, 321–339. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, J.; VIillalba, M.; Alvis-Dávila, L.; Garcia Marí, F. Identificación y abundancia de arañas (Araneae) en los cultivos de cítricos valencianos. Bol. Sanid. Veg. Plagas 2010, 36, 69–85. [Google Scholar]
- Michalko, R.; Pekár, S. The biocontrol potential of Philodromus (Araneae, Philodromidae) spiders for the suppression of pome fruit orchard pests. Biol. Control 2015, 82, 13–20. [Google Scholar] [CrossRef]
- Morris, T.; Symondson, W.; Kidd, N.; Campos Aranda, M. Las arañas y su incidencia sobre Prays oleae en el olivar. Boletín Sanid. Veg. Plagas 1999, 25, 475–489. [Google Scholar]
- Pekár, S. Some observations on overwintering of spiders (Araneae) in two contrasting orchards in the Czech Republic. Agric. Ecosyst. Environ. 1999, 73, 205–210. [Google Scholar] [CrossRef]
- Herrmann, J.D.; Bailey, D.; Hofer, G.; Herzog, F.; Schmidt-Entling, M.H. Spiders associated with the meadow and tree canopies of orchards respond differently to habitat fragmentation. Landsc. Ecol. 2010, 25, 1375–1384. [Google Scholar] [CrossRef]
- Pekár, S.; Michalko, R.; Loverre, P.; Líznarová, E.; Černecká, Ľ. Biological control in winter: Novel evidence for the importance of generalist predators. J. Appl. Ecol. 2014, 52, 270–279. [Google Scholar] [CrossRef]
- Petráková, L.; Michalko, R.; Loverre, P.; Sentenská, L.; Korenko, S.; Pekár, S. Intraguild predation among spiders and their effect on the pear psylla during winter. Agric. Ecosyst. Environ. 2016, 233, 67–74. [Google Scholar] [CrossRef]
- Michalko, R.; Petráková, L.; Sentenská, L.; Pekár, S. The effect of increased habitat complexity and density-dependent non-consumptive interference on pest suppression by winter-active spiders. Agric. Ecosyst. Environ. 2017, 242, 26–33. [Google Scholar] [CrossRef]
- Hanna, C.; Hanna, C. The lethal and sublethal effects of three pesticides on the striped lynx spider (Oxyopes salticus Hentz). J. Appl. Entomol. 2013, 137, 68–76. [Google Scholar] [CrossRef]
- Nobre, T.; Meierrose, C. The species composition, whitin-plant distribution, and possible predatory role of spiders (Araneae) in a vineyard in Southern Portugal. Ekol. Bratisl. 2000, 19, 193–200. [Google Scholar]
- Lockley, T.; Young, O.P. Prey of the striped lynx spider Oxyopes salticus (Araneae, Oxyopidae), on cotton in the delta area of Mississsippi. J. Arachnol. 1987, 14, 395–397. [Google Scholar]
- Agnew, C.W.; Smith, J.W. Ecology of spiders (Araneae) in a peanut agroecosystem. Environ. Entomol. 1989, 18, 30–42. [Google Scholar] [CrossRef]
- Cárdenas, M.; Ruano, F.; García, P.; Pascual, F.; Campos, M. Impact of agricultural management on spider populations in the canopy of olive trees. Biol. Control 2006, 38, 188–195. [Google Scholar] [CrossRef]
- Pérez-Guerrero, S.; Gelan-Begna, A.; Tamajón, R.; Vargas-Osuna, E. Potential predation of non-webbuilding spider assemblage on cotton pests Helicoverpa armigera and Spodoptera littoralis (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2013, 23, 335–347. [Google Scholar] [CrossRef]
- Pérez-Guerrero, S.; Gelan-Begna, A.; Vargas-Osuna, E. Impact of Cheiracanthium pelasgicum (Araneae: Miturgidae) and Chrysoperla carnea (Neuroptera: Chrysopidae) intraguild predation on the potential control of cotton pest Helicoverpa armigera (Lepidoptera: Noctuidae). Biocontrol Sci. Technol. 2014, 24, 216–228. [Google Scholar] [CrossRef]
- Pfannenstiel, R.S. Spider predators of lepidopteran eggs in south Texas field crops. Biol. Control 2008, 46, 202–208. [Google Scholar] [CrossRef]
- Mansour, F.; Richman, D.B.; Whitcomb, W.H. Spider management in agroecosystems: Habitat manipulation. Environ. Manag. 1983, 7, 43–49. [Google Scholar] [CrossRef]
- Benhadi-Marín, J.; Pereira, J.A.; Barrientos, J.A.; Bento, A.; Santos, S.A.P. Diversity of predaceous arthropods in the almond tree canopy in Northeastern Portugal: A methodological approach. Entomol. Sci. 2011, 14, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Horton, D.R.; Miliczky, E.R.; Broers, D.A.; Lewis, R.R.; Calkins, C.O. Numbers, diversity, and phenology of spiders (Araneae) overwintering in cardboard bands placed in pear and apple orchards of central Washington. Ann. Entomol. Soc. Am. 2001, 94, 405–414. [Google Scholar] [CrossRef]
- Coddington, J.A.; Young, L.H.; Coyle, F.A. Estimating spider species richness in a southern Appalachian cove hardwood forest. J. Arachnol. 1996, 24, 111–128. [Google Scholar]
- Cardoso, P.; Silva, I.; De Oliveira, N.G.; Serrano, A.R.M. Indicator taxa of spider (Araneae) diversity and their efficiency in conservation. Biol. Conserv. 2004, 120, 517–524. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Establishing reliable spider (Araneae, Araneidae and Thomisidae) assemblage sampling protocols: Estimation of species richness, seasonal coverage and contribution of juvenile data to species richness and composition. Acta Oecol. 2006, 30, 21–32. [Google Scholar] [CrossRef]
- Pekár, S. Spiders (Araneae) in the pesticide world: An ecotoxicological review. Pest Manag. Sci. 2012, 68, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, C.; Schumacher, K.; Freier, B. Araneae as indicators in low-input strategies in crop protection. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2008, 16, 301–304. [Google Scholar]
- Stark, J.D.; Banks, J.E. Population level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Tahir, H.M.; Noor, T.; Bhatti, M.F.; Bano, M.; Butt, A.; Alam, I.; Arshad, M.; Mukhtar, M.K.; Khan, S.Y.; Ahmed, K.R.; et al. Acetochlor application at field-rate compromises the locomotion of the jumping spider Plexippus paykulli (Araneae: Salticidae). Afr. J. Agric. Res. 2012, 7, 3329–3333. [Google Scholar]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of synthetic and botanical pesticides on beneficial insects. Agric. Sci. 2016, 7, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Pekár, S. Effect of IPM practices and conventional spraying on spider population dynamics in an apple orchard. Agric. Ecosyst. Environ. 1999, 73, 155–166. [Google Scholar] [CrossRef]
- Wisniewska, J.; Prokopy, R.J. Pesticide effect on faunal composition, abundance, and body length of spiders (Araneae) in apple orchards. Environ. Entomol. 1997, 26, 763–776. [Google Scholar] [CrossRef]
- Mansour, F.; Nentwig, W. Effects of agrochemical residues on four spider taxa: Laboratory methods for pesticide tests with web-building spiders. Phytoparasitica 1988, 16, 317–326. [Google Scholar] [CrossRef]
- Kakoki, S.; Kamimuro, T.; Tsuda, K.; Sakamaki, Y. Use of a lower-volume, surface pesticide spray conserves spider assemblages in a tea field. J. Econ. Entomol. 2018, 111, 1595–1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, F. A malathion-tolerant strain of the spider Cheiracanthium mildei and its response to chlorpyrifos. Phytoparasitica 1984, 12, 163–166. [Google Scholar] [CrossRef]
- Bajwa, W.I.; Aliniazee, M.T. Spider fauna in apple ecosystem of Western Oregon and its field susceptibility to chemical and microbial insecticides. J. Econ. Entomol. 2001, 94, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.A.; Dibble, J.E.; Flint, M.L.; Marer, P.J.; Guye, A. Managing Insects and Mites with Spray Oils; Division of Agriculture and Natural Resources, University of California: Oakland, CA, USA, 1991. [Google Scholar]
- Specht, H.B.; Dondale, C.D. Spider populations in New Jersey apple orchards. J. Econ. Entomol. 1960, 53, 810–814. [Google Scholar] [CrossRef]
- Pekár, S. Comparative analysis of passive defences in spiders (Araneae). J. Anim. Ecol. 2014, 83, 779–790. [Google Scholar] [CrossRef]
- Foelix, R. Biology of Spiders, 3rd ed.; Oxford University Press: New York, NY, USA, 2011. [Google Scholar]
- Bogya, S.; Markó, V. Effect of pest management systems on ground-dwelling spider assemblages in an apple orchard in Hungary. Agric. Ecosyst. Environ. 1999, 73, 7–18. [Google Scholar] [CrossRef]
- Li, D.; Jackson, R.R. How temperature affects development and reproduction in spiders: A review. J. Therm. Biol. 1996, 21, 245–274. [Google Scholar] [CrossRef]
- Finch, O.D.; Blick, T.; Schuldt, A. Macroecological patterns of spider species richness across Europe. Biodivers. Conserv. 2008, 17, 2849–2868. [Google Scholar] [CrossRef]





| Basic Guild | Guild | Family | LISO | CO |
|---|---|---|---|---|
| WEB BUILDERS | Space web builders | THERIDIIDAE | 116 | 98 |
| Orb weavers | ARANEIDAE | 80 | 81 | |
| ULOBORIDAE | 1 | 3 | ||
| Wandering sheet/Tangle weavers | LINYPHIIDAE | 39 | 50 | |
| WANDERING SPIDERS | Ambushers | PHILODROMIDAE | 448 | 204 |
| PISAURIDAE | 15 | 14 | ||
| THOMISIDAE | 55 | 55 | ||
| Stalkers | SALTICIDAE | 243 | 67 | |
| OXYOPIDAE | 175 | 230 | ||
| Foliage runners | CLUBIONIDAE | 1 | 0 | |
| CHEIRACANTHIIDAE | 197 | 49 | ||
| SPARASIDAE | 1 | 0 | ||
| Ground runners | GNAPHOSIDAE | 9 | 3 |
| Intensity of Spraying | Year | Interaction | ||||
|---|---|---|---|---|---|---|
| Family | χ2(1) | p-Value | χ2(2) | p-Value | χ2(2) | p-Value |
| Philodromidae | 9.253 | 0.002 | 2.158 | 0.340 | 1.023 | 0.600 |
| Oxyopidae | 1.992 | 0.158 | 13.656 | 0.001 | 1.682 | 0.431 |
| Salticidae | 20.570 | <0.001 | 15.422 | <0.001 | 1.493 | 0.474 |
| Cheiracanthiidae | 21.872 | <0.001 | 16.956 | <0.001 | 2.959 | 0.228 |
| Theridiidae | 0.930 | 0.335 | 39.648 | <0.001 | 0.630 | 0.730 |
| Araneidae | 0.373 | 0.541 | 7.803 | 0.020 | 2.045 | 0.360 |
| Thomisidae | 2.248 | 0.134 | 7.783 | 0.020 | 3.254 | 0.196 |
| Linyphiidae | 3.150 | 0.076 | 3.918 | 0.141 | 2.002 | 0.367 |
| Intensity of Spraying | Year | Interaction | ||||
|---|---|---|---|---|---|---|
| Guild | χ2(1) | p-Value | χ2(2) | p-Value | χ2(2) | p-Value |
| Ambushers | 6.991 | 0.008 | 2.951 | 0.229 | 2.588 | 0.274 |
| Stalkers | 2.232 | 0.135 | 12.258 | 0.002 | 1.160 | 0.560 |
| Foliage Runners | 35.732 | <0.001 | 29.338 | <0.001 | 5.8179 | 0.054 |
| Space web builders | 0.982 | 0.322 | 53.939 | <0.001 | 0.247 | 0.884 |
| Orb Weavers | 0.185 | 0.667 | 15.496 | <0.001 | 1.533 | 0.465 |
| Tangle Weavers | 0.148 | 0.701 | 6.038 | 0.049 | 3.329 | 0.189 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Pedro, L.; Ortín-Angulo, M.C.; Miñano, J.; López-Gallego, E.; Sanchez, J.A. Structure of the Assemblages of Spiders in Mediterranean Pear Orchards and the Effect of Intensity of Spraying. Insects 2020, 11, 553. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11090553
de Pedro L, Ortín-Angulo MC, Miñano J, López-Gallego E, Sanchez JA. Structure of the Assemblages of Spiders in Mediterranean Pear Orchards and the Effect of Intensity of Spraying. Insects. 2020; 11(9):553. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11090553
Chicago/Turabian Stylede Pedro, Luis, María Carmen Ortín-Angulo, Jesús Miñano, Elena López-Gallego, and Juan Antonio Sanchez. 2020. "Structure of the Assemblages of Spiders in Mediterranean Pear Orchards and the Effect of Intensity of Spraying" Insects 11, no. 9: 553. https://0-doi-org.brum.beds.ac.uk/10.3390/insects11090553