The Influence of Planting Periods on Herbivore and Natural Enemy Abundance on Yellow Sticky Traps in Bt Maize Fields
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Cultivation Practices
2.2. Sampling of Herbivores and NEs
2.3. Statistical Analysis
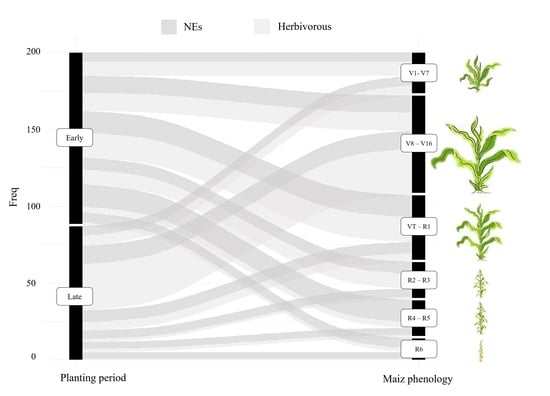
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maresma, A.; Ballesta, A.; Santiveri, F.; Lloveras, J. Sowing date affects maize development and yield in irrigated mediterranean environments. Agriculture 2019, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Tsimba, R.; Edmeades, G.O.; Millner, J.P.; Kemp, P.D. The effect of planting date on maize grain yields and yield components. Field Crop. Res. 2013, 150, 135–144. [Google Scholar] [CrossRef]
- Hall, R.G.; Reitsma, K.D.; Clay, D.E. Best management practices for corn production in South Dakota: Corn planting guide. In Grow Corn: Best Management Practices; South Dakota State University: Brookings, SD, USA, 2016; Chapter 3; pp. 13–16. [Google Scholar]
- Kucharik, C.J. A multidecadal trend of earlier corn planting in the central USA. Agron. J. 2006, 98, 1544–1550. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Agricultura, Alimentación y Medio Ambiente. Guía de Gestión Integrada de Plagas. Maíz. 2015. Available online: https://www.mapa.gob.es/es/agricultura/temas/sanidad-vegetal/guiamaiz_tcm30-57958.pdf (accessed on 20 January 2017).
- Albajes, R.; Lumbierres, B.; Pons, X.; Comas, J. Representative taxa in field trials for environmental risk assessment of genetically modified maize. Bull. Entomol. Res. 2013, 10, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achon, M.A.; Subira, J.; Sin, E. Seasonal occurrence of Laodelphax striatellus in Spain: Effect on the incidence of Maize rough dwarf virus. Crop Prot. 2013, 47, 1–5. [Google Scholar] [CrossRef]
- Achon, M.A.; Serrano, L.; Sabaté, L.; Porta, C. Understanding the epidemiological factors that intensify the incidence of maize rough dwarf disease in Spain. Ann. Appl. Biol. 2015, 16, 311–320. [Google Scholar] [CrossRef]
- Achon, M.A.; Sobrepere, M. Incidence of potyviruses in commercial maize fields and their seasonal cycles in Spain. Zeit. Pflan. Pflanzenschutz. 2001, 108, 399–406. [Google Scholar]
- Clemente-Orta, G.; Madeira, F.; Batuecas, I.; Sossai, S.; Juárez-Escario, A.; Albajes, R. Changes in landscape composition influence the abundance of insects on maize: The role of fruit orchards and alfalfa crops. Agric. Ecosyst. Environ. 2020, 291, 106805. [Google Scholar] [CrossRef]
- Clemente-Orta, G.; Albajes, R.; Achon, M.A. Early planting, management of edges and non-crop habitats reduce potyvirus infection in maize. Agron. Sustain. 2020, 40, 21. [Google Scholar] [CrossRef]
- Clemente-Orta, G.; Albajes, R.; Batuecas, I.; Achon, M.A. Planting period is the main factor for controlling maize rough dwarf disease. Sci. Rep. 2021, 11, 977. [Google Scholar] [CrossRef]
- Ritchie, S.W.; Hanway, J.J.; Benson, G.O. How a Corn Plant Develops; Iowa State University: Ames, IA, USA, 1992. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Liliane, T.N.; Charles, M.S. Factors affecting yield of crops. In Agronomy: Climate Change & Food Security; IntechOpen: London, UK, 2020; Volume 9. [Google Scholar]
- Batyrshina, Z.S.; Cna’ani, A.; Rozenberg, T.; Seifan, M.; Tzin, V. The combined impacts of wheat spatial position and phenology on cereal aphid abundance. PeerJ 2020, 8, e9142. [Google Scholar] [CrossRef] [PubMed]
- Nboyine, J.A.; Kusi, F.; Abudulai, M.; Badii, B.K.; Zakaria, M.; Adu, G.B.; Yahaya, A. A new pest, Spodoptera frugiperda (JE Smith), in tropical Africa: Its seasonal dynamics and damage in maize fields in northern Ghana. Crop Prot. 2020, 127, 104960. [Google Scholar] [CrossRef]
- Ouali-N’goran, S.M.; Sylvain, T.B.C.; Adamou, U.D.M.; Yao, T.A.N.O. Diversity and abundance of insect pests of corn (Zea mays Poaceae) grown in a rural environment in the city of MBahiakro (East Central Cte dIvoire). J. Ecol. Nat. Environ. 2017, 9, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, G.C.D.; Martins, I.C.F.; Campos, L.D.; Mello, M.N.; Mejdalani, G. Spatial and temporal distribution of leafhoppers (Hemiptera: Cicadellidae) in a corn field. Neotrop. Entomol. 2021, 50, 630–642. [Google Scholar] [CrossRef]
- Luft Albarracin, E.; Paradell, S.; Virla, E.G. Cicadellidae (Hemiptera: Auchenorrhyncha) associated with maize crops in northwestern Argentina. Influence of the sowing date and phenology of their abundance and diversity. Maydica 2008, 53, 289–296. [Google Scholar]
- Bastos, C.S.; Galvão, J.C.C.; Picanço, M.C.; Cecon, P.R.; Pereira, P.R.G. Incidência de insetos fitófagos e de predadores no milho e no feijão cultivados em sistema exclusivo e consorciado. Ciênc. Rural 2003, 33, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Maia, W.J.M.S.; Cruz, I.; Carvalho, C.F.; Souza, B.; Waquil, J.M.; Von Pinho, R.G.; Carvalho, S.P.; Maia, T.J.A.F.; Loureiro, I. Efeito do estádio fenológico do milho (Zea mays L.) sobre a infestação pelo pulgão Rhopalosiphum maidis (Fitch, 1865). Rev. Bras. Milho Sorgo 2005, 4, 308–315. [Google Scholar] [CrossRef]
- Gutierrez, C.; Castañera, P. Efecto de los tejidos de maiz con alto y bajo contenido en dimboa sobre, la biologia del taladro Sesamia nonagrioides Lef. (Lepidoptera, Noctuidae). Investig. Agrar. Prod. Prot. Veg. 1986, 1, 109–119. [Google Scholar]
- Barry, D.; Alfaro, D.; Darrah, L.L. Relation of European corn borer (Lepidoptera:Pyralidae) leaf-feeding resistance and DIMBOA content in maize. Environ. Entomol. 1994, 23, 177–182. [Google Scholar] [CrossRef]
- Thackray, D.J.; Wratten, S.D.; Edwards, P.J.; Niemeyer, H.M. Resistance to the aphids Sitobion avenae and Rhopalosiphum padi in Gramineae in relation to hydroxamic acid levels. Ann. Appl. Biol. 1990, 116, 573–582. [Google Scholar] [CrossRef]
- Cambier, V.; Hance, T.; de Hoffmann, E. Variation of DIMBOA and related compounds content in relation to the age and plant organ in maize. Phytochemistry 2000, 53, 223–229. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Hydroxamic acids (4-hydroxy-1, 4-benzoxazin-3-ones), defence chemicals in the Gramineae. Phytochemistry 1988, 27, 3349–3358. [Google Scholar] [CrossRef]
- Albajes, R.; Lumbierres, B.; Pons, X.; Comas, J. Changes in arthropod fauna from weed management practices in genetically modified herbicide-tolerant maize. J. Agric. Sci. 2014, 6, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Ardanuy, A.; Lee, M.S.; Albajes, R. Landscape context influences leafhopper and predatory Orius spp. abundances in maize fields. Agric. For. Entomol. 2018, 20, 81–92. [Google Scholar] [CrossRef]
- Pan, H.; Xiu, C.; Liu, B.; Wyckhuys, K.A.; Lu, Y. Whorl-stage maize provides a microclimate refuge for predatory ladybeetles. Biol. Control 2020, 142, 104162. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Westphal, C. Landscape moderation of biodiversity patterns and processes-eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]




| Herbivore | Variable | Chisq | Df | p Value |
|---|---|---|---|---|
| F. occidentalis | Planting | 1.437 | 1 | 0.23 |
| Phenology | 147.29 | 5 | <0.001 | |
| Planting:Phenology | 210.84 | 5 | <0.001 | |
| “Other herbivore thrips” | Planting | 12.62 | 1 | <0.001 |
| Phenology | 494.35 | 5 | <0.001 | |
| Z. scutellaris | Planting | 0.77 | 1 | 0.38 |
| Phenology | 160.92 | 5 | <0.001 | |
| Empoasca sp. | Planting | 0.201 | 1 | 0.65 |
| Phenology | 18.08 | 5 | 0.002 | |
| Planting:Phenology | 11.98 | 5 | 0.03 | |
| Aphids | Planting | 3.31 | 1 | 0.06 |
| Phenology | 564.93 | 5 | <0.001 | |
| L. striatellus | Planting | 0.42 | 1 | 0.51 |
| Phenology | 66.36 | 5 | <0.001 | |
| Planting:Phenology | 15.3 | 5 | 0.009 |
| Natural Enemy | Variable | Chisq | Df | p Value |
|---|---|---|---|---|
| Aeolothrips sp. | Planting | 0.01 | 1 | 0.89 |
| Phenology | 207.51 | 5 | <0.001 | |
| Syrphidae | Planting | 0.50 | 1 | 0.47 |
| Phenology | 79.73 | 5 | <0.001 | |
| Planting:Phenology | 14.72 | 5 | 0.01 | |
| Orius spp. | Planting | 2.3 | 1 | 0.12 |
| Phenology | 82.98 | 5 | <0.001 | |
| Chrysopidae | Planting | 1.97 | 1 | 0.16 |
| Phenology | 10.28 | 5 | 0.06 | |
| Coccinellidae | Planting | 1.27 | 1 | 0.25 |
| Phenology | 25.63 | 5 | <0.001 | |
| S. punctillum | Planting | 0.22 | 1 | 0.63 |
| Phenology | 134.75 | 5 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente Orta, G.; Álvarez, H.A.; Madeira, F.; Albajes, R. The Influence of Planting Periods on Herbivore and Natural Enemy Abundance on Yellow Sticky Traps in Bt Maize Fields. Insects 2022, 13, 388. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040388
Clemente Orta G, Álvarez HA, Madeira F, Albajes R. The Influence of Planting Periods on Herbivore and Natural Enemy Abundance on Yellow Sticky Traps in Bt Maize Fields. Insects. 2022; 13(4):388. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040388
Chicago/Turabian StyleClemente Orta, Gemma, Hugo Alejandro Álvarez, Filipe Madeira, and Ramon Albajes. 2022. "The Influence of Planting Periods on Herbivore and Natural Enemy Abundance on Yellow Sticky Traps in Bt Maize Fields" Insects 13, no. 4: 388. https://0-doi-org.brum.beds.ac.uk/10.3390/insects13040388