Interaction Networks Help to Infer the Vulnerability of the Saproxylic Beetle Communities That Inhabit Tree Hollows in Mediterranean Forests
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
2.2. Climate Trends
2.3. Diversity Analysis
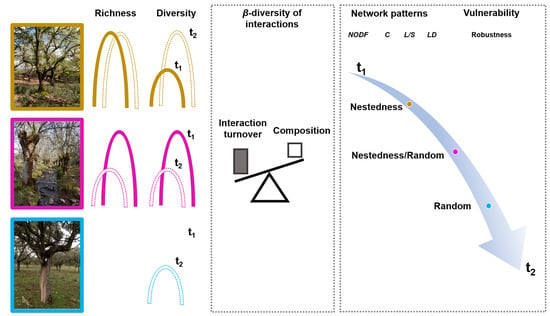
2.4. Network Patterns and Interacting Attributes
2.5. Interannual Beta Diversity of Interactions
2.6. Microhabitat Loss Simulations
3. Results
3.1. Long-Term Variation in Diversity Patterns
3.2. Interannual Changes in Network Patterns and Interacting Attributes
3.3. Network Beta Diversity
3.4. Vulnerability to Microhabitat Loss
4. Discussion
4.1. Interannual Changes in Network and Diversity Patterns
4.2. Temporal Beta Diversity of the Tree Hollow–Saproxylic Beetle Interaction
4.3. Saproxylic Communities’ Vulnerability in a Changing World
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shortall, C.R.; Moore, A.; Smith, E.; Hall, M.J.; Woiwod, I.P.; Harrington, R. Long-term changes in the abundance of flying insects. Insect Conserv. Divers. 2009, 2, 251–260. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Dirnböck, T.; Müller, J.; Kobler, J.; Katzensteiner, K.; Helm, N.; Seidl, R. The impacts of climate change and disturbance on spatio-temporal trajectories of biodiversity in a temperate forest landscape. J. Appl. Ecol. 2017, 54, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Didham, R.K.; Barbero, F.; Collins, C.M.; Forister, M.L.; Hassall, C.; Leather, S.R.; Packer, L.; Saunders, M.E.; Stewart, A.J.A. Spotlight on insects: Trends, threats and conservation challenges. Insect Conserv. Divers. 2020, 13, 99–102. [Google Scholar] [CrossRef]
- Wagner, D.L.; Fox, R.; Salcido, D.M.; Dyer, L.A. A window to the world of global insect declines: Moth biodiversity trends are complex and heterogeneous. Proc. Natl. Acad. Sci. USA 2020, 118, e2002549117. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Proschwitz, T.V.; Fägerström, C.; Green, M.; Smith, H.G.; Lindström, A. Arthropod populations in a sub-arctic environment facing climate change over a half-century: Variability but no general trend. Insect Conserv. Divers. 2022, 15, 534–542. [Google Scholar] [CrossRef]
- Blumgart, D.; Botham, M.S.; Menéndez, R.; Bell, J.R. Moth declines are most severe in broadleaf woodlands despite a net gain in habitat availability. Insect Conserv. Divers. 2022, 15, 496–509. [Google Scholar] [CrossRef]
- Conrad, K.F.; Warren, M.S.; Fox, R.; Parsons, M.S.; Woiwod, I.P. Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol. Conserv. 2006, 132, 279–291. [Google Scholar] [CrossRef]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Ssymankb, A.; Sorgc, M.; de Kroon, H.; Jongejans, E. Insect biomass decline scaled to species diversity: General patterns derived from a hoverfly community. Proc. Natl. Acad. Sci. USA 2021, 118, e2002554117. [Google Scholar] [CrossRef]
- Janzen, D.H.; Hallwachs, W. To us insectometers, it is clear that insect decline in our Costa Rican tropics is real, so let’s be kind to the survivors. Proc. Natl. Acad. Sci. USA 2021, 118, e2002546117. [Google Scholar] [CrossRef]
- Wilson, R.J.; Fox, R. Insect responses to global change offer signposts for biodiversity and conservation. Ecol. Entomol. 2021, 46, 699–717. [Google Scholar] [CrossRef]
- Winfree, R.W.; Fox, J.; Williams, N.M.; Reilly, J.R.; Cariveau, D.P. Abundance of common species, not species richness, drives delivery of a real-world ecosystem service. Ecol. Lett. 2015, 18, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, R.; Frago, E.; Sanders, D. Cascading extinctions as a hidden driver of insect decline. Ecol. Entomol. 2021, 46, 743–756. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Lister, B.; Garcia, A. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl. Acad. Sci. USA 2018, 115, e10397–e10406. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Valladares, F.; Cantera, X.; Escudero, A. La Salud Planetaria; CSIC: Madrid, Spain, 2022; pp. 1–140. [Google Scholar]
- Chowdhury, S.; Jennions, M.D.; Zalucki, M.P.; Maron, M.; Watson, J.E.M.; Fuller, R.A. Protected areas and the future of insect conservation. Trends Ecol. Evol. 2023, 38, 85–95. [Google Scholar] [CrossRef]
- Chowdhury, S.; Zalucki, M.P.; Hanson, J.O.; Tiatragul, S.; Green, D.; Watson, J.E.M.; Fuller, R.A. Three-quarters of insect species are insufficiently represented by protected areas. One Earth 2023, 6, 139–146. [Google Scholar] [CrossRef]
- Habel, J.C.; Samways, M.J.; Schmitt, T. Mitigating the precipitous decline of terrestrial European insects: Requirements for a new strategy. Biodivers. Conserv. 2019, 28, 1343–1360. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists' warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Yang, L.H.; Postema, E.G.; Hayes, T.E.; Lippey, M.K.; MacArthur-Waltz, D.J. The complexity of global change and its effects on insects. Curr. Opin. Insect Sci. 2021, 47, 90–102. [Google Scholar] [CrossRef]
- Brooks, D.R.; Bater, J.E.; Clark, S.J.; Monteith, D.T.; Andrews, C.; Corbett, S.J.; Beaumont, D.A.; Chapman, J.W. Large carabid beetle declines in a United Kingdom monitoring network increases evidence for a widespread loss in insect biodiversity. J. Appl. Ecol. 2012, 49, 1009–1019. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Zeegers, T.; van Klink, R.; Vermeulen, R.; van Wielink, P.; Spijkers, H.; van Deijk, J.; van Steenis, W.; Jongejans, E. Declining abundance of beetles, moths and caddisflies in The Netherlands. Insect Conserv. Divers. 2020, 13, 127–139. [Google Scholar] [CrossRef]
- Beiroz, W.; Slade, E.M.; Barlow, J.; Silveira, J.M.; Louzada, J.; Sayer, E. Dung beetle community dynamics in undisturbed tropical forests, implications for ecological evaluations of land-use change. Insect Conserv. Divers. 2017, 10, 94–106. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (Version 1). Zenodo. 2019. Available online: https://www.ipbes.net/global-assessment (accessed on 20 March 2023).
- Montoya, J.M.; Pimm, S.L.; Solé, R.V. Ecological networks and their fragility. Nature 2006, 442, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Castelazo, C.; Guimarães, P.R., Jr.; Jordano, P.; Thompson, J.N.; Marquis, R.J.; Rico-Gray, V. Changes of a mutualistic network over time: Reanalysis over a 10-year period. Ecology 2010, 91, 793–801. [Google Scholar] [CrossRef]
- Villa-Galaviz, E.; Boege, K.; Del-Val, E. Resilience in plant-herbivore networks during secondary succession. PLoS ONE 2012, 7, e53009. [Google Scholar] [CrossRef]
- Saavedra, S.; Cenci, S.; Del-Val, E.; Boege, K.; Rohr, R.P. Reorganization of interaction networks modulates the persistence of species in late successional stages. J. Anim. Ecol. 2017, 86, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Quinto, J.; Díaz-Castelazo, C.; Rico-Gray, V.; Martínez-Falcón, A.P.; Abdala-Roberts, L.; Parra-Tabla, V. Short-term temporal patterns in herbivore beetle assemblages in polyculture Neotropical forest plantations. Neotrop. Entomol. 2022, 51, 199–211. [Google Scholar] [CrossRef]
- Carstensen, D.W.; Sabatino, M.; Trøjelsgaard, K.; Morellato, L.P.C. Beta diversity of plant-pollinator networks and the spatial turnover of pairwise interactions. PLoS ONE 2014, 9, e112903. [Google Scholar] [CrossRef]
- Martínez-Falcón, A.P.; Martínez-Adriano, C.A.; Dáttilo, W. Redes complejas como herramientas para estudiar la diversidad de las interacciones ecológicas. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos Para su Estudio; Moreno, C.E., Ed.; Universidad Autónoma del Estado de Hidalgo/Libermex: Ciudad de México, Mexico, 2019; pp. 265–283. [Google Scholar]
- Petsopoulos, D.; Lunt, D.H.; Bell, J.R.; Kitson, J.J.N.; Collins, L.; Boonham, N.; Morales-Hojas, R.; Evans, D.M. Using network ecology to understand and mitigate long-term insect declines. Ecol. Entomol. 2021, 46, 693–698. [Google Scholar] [CrossRef]
- Borrvall, C.; Ebenman, B.; Jonsson, T. Biodiversity lessens the risk of cascading extinction in model food webs. Ecol. Lett. 2000, 3, 131–136. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M.; Price, M.V. Tolerance of pollinator networks to species extinctions. Proc. R. Soc. B Biol. Sci. 2004, 271, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Dunne, J.A.; Williams, R.J. Cascading extinctions and community collapse in model food webs. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Quinto, J.; Micó, E.; Marcos-García, M.A.; Martínez-Falcón, A.P.; Galante, E. Influence of tree hollow microenvironmental variables on saproxylic guild diversity in Iberian Mediterranean woodland. J. Insect Conserv. 2014, 18, 981–992. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Šobotník, J. An introduction to the diversity, ecology, and conservation of saproxylic insects. In Saproxylic Insects. Zoological Monographs; Ulyshen, M., Ed.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 1–47. [Google Scholar]
- Müller, J.; Jarzabek-Müller, A.; Bussler, H.; Gossner, M.M. Hollow beech trees identified as keystone structures for saproxylic beetles by analyses of functional and phylogenetic diversity. Anim. Conserv. 2014, 17, 154–162. [Google Scholar] [CrossRef]
- Micó, E. Saproxylic insects in tree hollows. In Saproxylic Insects. Zoological Monographs; Ulyshen, M., Ed.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 693–797. [Google Scholar]
- Henneberg, B.; Bauer, S.; Birkenbach, M.; Mertl, V.; Steinbauer, M.J.; Feldhaar, H.; Obermaier, E. Influence of tree hollow characteristics and forest structure on saproxylic beetle diversity in tree hollows in managed forests in a regional comparison. Ecol. Evol. 2021, 11, 17973–17999. [Google Scholar] [CrossRef]
- Oleksa, A.; Chybicki, I.J.; Gawroński, R.; Svensson, G.P.; Burczyk, J. Isolation by distance in saproxylic beetles may increase with niche specialization. J. Insect Conserv. 2013, 17, 219–233. [Google Scholar] [CrossRef]
- Quinto, J.; Marcos-García, M.A.; Brustel, H.; Galante, E.; Micó, E. Effectiveness of three sampling methods to survey saproxylic beetle assemblages. J. Insect Conserv. 2013, 17, 765–776. [Google Scholar] [CrossRef]
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting insect declines: Seven challenges and a way forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Gough, L.A.; Sverdrup-Thygeson, A.; Milberg, P.; Pilskog, H.E.; Jansson, N.; Jonsell, M.; Birkemoe, T. Specialists in ancient trees are more affected by climate than generalists. Ecol. Evol. 2015, 5, 5632–5641. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Rodenhouse, N.L.; Holmes, R.T. Decline in beetle abundance and diversity in an intact temperate forest linked to climate warming. Biol. Conserv. 2019, 240, 108219. [Google Scholar] [CrossRef]
- Kozák, D.; Svitok, M.; Wiezik, M.; Mikoláš, M.; Thorn, S.; Buechling, A.; Hofmeister, J.; Matula, R.; Trotsiuk, V.; Bače, R.; et al. Historical disturbances determine current taxonomic, functional and phylogenetic diversity of saproxylic beetle communities in temperate primary forests. Ecosystems 2021, 24, 37–55. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Lozano-García, B.; Muñoz-Rojas, M.; Parras-Alcántara, L. Climate and land use changes effects on soil organic carbon stocks in a Mediterranean semi-natural area. Sci. Total Environ. 2017, 579, 1249–1259. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Emmett, B.A.; Britton, A.J.; Caporn, S.J.; Dise, N.B.; Helliwell, R.; Jones, L.; Leake, J.R.; Leith, I.D.; Sheppard, L.J.; et al. Impacts of atmospheric nitrogen deposition: Responses of multiple plant and soil parameters across contrasting ecosystems in long-term field experiments. Glob. Chang Biol. 2012, 18, 1197–1215. [Google Scholar] [CrossRef]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Quinto, J.; Marcos-García, M.A.; Díaz-Castelazo, C.; Rico-Gray, V.; Galante, E.; Micó, E. Association patterns in saproxylic insect networks in three Mediterranean woodlands and resistance to microhabitat loss. PLoS ONE 2015, 10, e0122141. [Google Scholar] [CrossRef]
- Lange, D.; Del-Claro, K. Ant-plant interaction in a tropical savanna: May the network structure vary over time and influence on the outcomes of associations? PLoS ONE 2014, 9, e105574. [Google Scholar] [CrossRef]
- Traill, L.W.; Lim, M.L.M.; Sodhi, N.S.; Bradshaw, C.J.A. Mechanisms driving change: Altered species interactions and ecosystem function through global warming. J. Anim. Ecol. 2010, 79, 937–947. [Google Scholar] [CrossRef]
- Windsor, F.M.; van den Hoogen, J.; Crowther, T.W.; Evans, D.M. Using ecological networks to answer questions in global biogeography and ecology. J. Biogeogr. 2023, 50, 57–69. [Google Scholar] [CrossRef]
- Evans, D.M.; Pocock, M.J.O.; Memmott, J. The robustness of a network of ecological networks to habitat loss. Ecol. Lett. 2013, 16, 844–852. [Google Scholar] [CrossRef]
- Ministerio para la Transición Ecológica y el Reto Demográfico, 2021. Cabañeros National Park General Information. Available online: https://www.miteco.gob.es/es/red-parques-nacionales/nuestros-parques/cabaneros/informaciongeneral2021_tcm30-62021.pdf (accessed on 10 April 2023).
- Sánchez-Galván, I.R.; Quinto, J.; Micó, E.; Galante, E.; Marcos-García, M.A. Facilitation among saproxylic insects inhabiting tree-hollows in a Mediterranean forest: The case of cetonids (Coleoptera: Cetoniidae) and syrphids (Diptera: Syrphidae). Environ. Entomol. 2014, 43, 336–343. [Google Scholar] [CrossRef]
- Audisio, P.; Baviera, C.; Carpaneto, G.M.; Biscaccianti, A.B.; Battistoni, A.; Teofili, C.; Rondinini, C. Lista Rossa IUCN dei Coleotteri saproxilici Italiani; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2014; pp. 1–132. [Google Scholar]
- Bouget, C.; Brustel, H.; Noblecourt, T.; Zagatti, P. Les Coléoptères saproxyliques de France; In Catalogue Écologique Illustré; Muséum National D’histoire Naturelle: Paris, France, 2019; pp. 1–744. [Google Scholar]
- Micó, E.; Marcos-García, M.A.; Ramírez-Hernández, A.; Galante, E. El Bosque Adehesado Como Refugio de una Entomofauna Muy Diversa; Publicaciones de la Universitat d’Alacant: Alicante, Spain, 2021; pp. 1–143. [Google Scholar]
- AEMET. Agencia Estatal de Meteorología—AEMET. Available online: https://opendata.aemet.es/centrodedescargas/inicio (accessed on 2 November 2022).
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Moreno, C.E.; Calderón-Patrón, J.M.; Arroyo-Rodríguez, V.; Barragán, F.; Escobar, F.; Gómez-Ortiz, Y.; Martín-Regalado, N.; Martínez-Falcón, A.P.; Martínez-Morales, M.A.; Mendoza, E.; et al. Measuring biodiversity in the Anthropocene: A simple guide to helpful methods. Biol. Conserv. 2017, 26, 2993–2998. [Google Scholar] [CrossRef]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: iNterpolation and EXTrapolation for Species Diversity. R package version 2.0.19. 2019. Available online: http://chao.stat.nthu.edu.tw/blog/software-download/ (accessed on 20 June 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 23 May 2022).
- Clarke, K.R.; Gorley, R.N. PRIMER v7; PRIMER-E: Plymouth, UK, 2015. Available online: https://www.primer-e.com/our-software/primer-version-7/ (accessed on 28 June 2022).
- Güimarães, P.R., Jr.; Güimarães, P. Improving the analyses of nestedness for large sets of matrices. Environ. Model. Softw. 2006, 21, 1512–1513. [Google Scholar] [CrossRef]
- Güimarães, P.R., Jr.; Rico-Gray, V.; Reis, S.F.; Thompson, J.N. Asymmetries in specialization in ant-plant mutualistic networks. Proc. R. Soc. B Biol. Sci. 2006, 273, 2041–2047. [Google Scholar] [CrossRef]
- Atmar, W.; Patterson, B.D. The measure of order and disorder in the distribution of species in fragmented habitat. Oecologia 1993, 96, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Marquitti, F.M.D.; Guimarães, P.R.; Pires, M.M.; Bittencourt, L.F. MODULAR: Software for the autonomous computation of modularity in large network sets. Ecography 2014, 37, 221–224. [Google Scholar] [CrossRef]
- Guimerà, R.; Stoufer, D.B.; Sales-Pardo, M.; Leicth, E.A.; Newman, M.E.J.; Amaral, L.A.N. Origin of compartmentalization in food webs. Ecology 2010, 91, 2941–2951. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 8–11. [Google Scholar]
- Vázquez, D.P.; Melián, C.J.; Williams, N.M.; Blüthgen, N.; Krasnov, B.R.; Poulin, R. Species abundance and asymmetric interaction strength in ecological networks. Oikos 2007, 116, 1120–1127. [Google Scholar] [CrossRef]
- Poisot, T.; Canard, E.; Mouillot, D.; Mouquet, N.; Gravel, D. The dissimilarity of species interaction networks. Ecol. Lett. 2012, 15, 1353–1361. [Google Scholar] [CrossRef]
- Burgos, E.; Ceva, H.; Perazzo, R.P.J.; Devoto, M.; Medan, D.; Zimmermann, M.; Delbue, M. Why nestedness in mutualistic networks? J. Theor. Biol. 2007, 249, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Martín González, A.M.; Vázquez, D.P.; Ramos-Jiliberto, R.; Lee, S.H.; Miele, V. Core–periphery structure in mutualistic networks: An epitaph for nestedness? bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Dáttilo, W.; Guimarães, P.R., Jr.; Izzo, T.J. Spatial structure of ant–plant mutualistic networks. Oikos 2013, 122, 1643–1648. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Olesen, J.M. Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science 2006, 312, 431–433. [Google Scholar] [CrossRef]
- López-Carretero, A.; Boege, K.; Díaz-Castelazo, C.; Domínguez, Z.; Rico-Gray, V. Influence of plant resistance traits in selectiveness and species strength in a tropical plant-herbivore network. Am. J. Bot. 2016, 103, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Bascompte, J.; Jordano, P.; Melián, C.J.; Olesen, J.M. The nested assembly of plant-animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Hernández, A.; Martínez-Falcón, A.P.; Micó, E.; Almendarez, S.; Reyes-Castillo, P.; Escobar, F. Diversity and deadwood-based interaction networks of saproxylic beetles in remnants of riparian cloud forest. PLoS ONE 2019, 14, e0214920. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.; Möller, G.C. Microhabitats in lowland beech forests as monitoring tool for nature conservation. For. Ecol. Manag. 2008, 255, 1251–1261. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Micó, E.; Galante, E. Temporal variation in saproxylic beetle assemblages in a Mediterranean ecosystem. J. Insect Conserv. 2014, 18, 993–1007. [Google Scholar] [CrossRef]
- Ramos-Robles, M.; Vargas-Cardoso, O.R.; Corona-López, A.M.; Flores-Palacios, A.; Toledo-Hernández, V.H. Spatio-temporal variation of Cerambycidae-host tree interaction networks. PLoS ONE 2020, 15, e0228880. [Google Scholar] [CrossRef]
- Galiana, N.; Lurgi, M.; Bastazini, V.A.G.; Bosch, J.; Cagnolo, L.; Cazelles, K.; Claramunt-López, B.; Emer, C.; Fortin, M.J.; Grass, I.; et al. Ecological network complexity scales with area. Nat. Ecol. Evol. 2022, 6, 307–314. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R.; Loyola, R.D.; Urlich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Cantor, M.; Pires, M.M.; Marquitti, F.M.D.; Raimundo, R.L.G.; Sebastián-González, E.; Coltri, P.P.; Perez, S.I.; Barneche, D.R.; Brandt, D.Y.C.; Nunes, K.; et al. Nestedness across biological scales. PLoS ONE 2017, 12, e0171691. [Google Scholar] [CrossRef]
- Dáttilo, W.; Vizentin-Bugoni, J.; Debastiani, V.J.; Jordano, P.; Izzo, T.J. The influence of spatial sampling scales on ant–plant interaction network architecture. J. Anim. Ecol. 2019, 88, 903–914. [Google Scholar] [CrossRef]
- Schwarz, B.; Vázquez, D.P.; CaraDonna, P.J.; Knight, T.M.; Benadi, G.; Dormann, C.F.; Gauzens, B.; Motivans, E.; Resasco, J.; Blüthgen, N.; et al. Temporal scale-dependence of plant–pollinator networks. Oikos 2020, 129, 1289–1302. [Google Scholar] [CrossRef]
- Baumgartner, M.T. Connectance and nestedness as stabilizing factors in response to pulse disturbances in adaptative antagonistic networks. J. Theor. Biol. 2020, 486, 110073. [Google Scholar] [CrossRef]
- Bain, J.A.; Dickson, R.G.; Gruver, A.M.; CaraDonna, P.J. Removing flowers of a generalist plant changes pollinator visitation, composition, and interaction network structure. Ecosphere 2022, 13, e4154. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lewinsohn, T.M.; Kahl, T.; Grassein, F.; Boch, S.; Prati, D.; Birkhofer, K.; Renner, S.C.; Sikorski, J.; Wubet, T.; et al. Land-use intensification causes multitrophic homogenization of grassland communities. Nature 2016, 540, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Sala, D.; Sabaté, S.; Sánchez-Costa, E.; Poblador, S.; Sabater, F.; Gracia, C. Growth and water use performance of four co-occurring riparian tree species in a Mediterranean riparian forest. For. Ecol. Manag. 2017, 396, 132–142. [Google Scholar] [CrossRef]
- Rada, P.; Padilla, A.; Horák, J.; Micó, E. Public LiDAR data are an important tool for the detection of saproxylic insect hotspots in Mediterranean forests and their connectivity. For. Ecol. Manag. 2022, 520, 120378. [Google Scholar] [CrossRef]
- Evans, M.J.; Barton, P.; Niwa, S.; Soga, M.; Seibold, S.; Tsuchiya, K.; Hisano, M. Climate-driven divergent long-term trends of forest beetles in Japan. Ecol. Lett. 2022, 25, 2009–2021. [Google Scholar] [CrossRef] [PubMed]
- de Marco, A.; Sicard, P.; Feng, Z.; Agathokleous, E.; Alonso, R.; Araminiene, V.; Augustatis, A.; Badea, O.; Beasley, J.C.; Branquinho, C.; et al. Strategic roadmap to assess forest vulnerability under air pollution and climate change. Glob. Chang Biol. 2022, 28, 5062–5085. [Google Scholar] [CrossRef]
- Villanueva, F.; Tapia, A.; Notario, A.; Albaladejo, J.; Martínez, E. Ambient levels and temporal trends of VOCs, including carbonyl compounds, and ozone at Cabañeros National Park border, Spain. Atmos. Environ. 2014, 85, 256–265. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Tamir Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Steven Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- Lindman, L.; Öckinger, E.; Ranius, T. Microclimate in hollow trees and how it affects an inhabiting beetle species, Osmoderma eremita. Ecol. Entomol. 2022, 48, 112–126. [Google Scholar] [CrossRef]
- Seibold, S.; Hagge, J.; Müller, J.; Gruppe, A.; Brandl, R.; Bässler, C.; Thorn, S. Experiments with dead wood reveal the importance of dead branches in the canopy for saproxylic beetle conservation. For. Ecol. Manag. 2018, 409, 564–570. [Google Scholar] [CrossRef]
- Dunne, A.J.; Williams, J.R.; Martinez, D.N. Network structure and biodiversity loss in food webs: Robustness increases with connectance. Ecol. Lett. 2002, 5, 558–567. [Google Scholar] [CrossRef]
- Sallé, A.; Cours, J.; Le Souchu, E.; Lopez-Vaamonde, C.; Pincebourde, S.; Bouget, C. Climate change alters temperate forest canopies and indirectly reshapes arthropod communities. Front. For. Glob. Change 2021, 4, 710854. [Google Scholar] [CrossRef]
- Landi, P.; Minoarivelo, H.O.; Brännström, Å.; Hui, C.; Dieckmann, U. Complexity and stability of ecological networks: A review of the theory. Popul. Ecol. 2018, 60, 319–345. [Google Scholar] [CrossRef]
- Thébault, E.; Fontaine, C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 2010, 329, 853–856. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Evans, D.M.; Memmott, J. The robustness and restoration of a network of ecological networks. Science 2012, 335, 973–977. [Google Scholar] [CrossRef]
- Della Rocca, F.; Bogliani, G.; Breiner, F.T.; Milanesi, P. Identifying hotspots for rare species under climate change scenarios: Improving saproxylic beetle conservation in Italy. Biodivers. Conserv. 2019, 28, 433–449. [Google Scholar] [CrossRef]





| Park Scale | Deciduous Woodland | Riparian Woodland | Sclerophyllous Woodland | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2021 | A | B | 2009 | 2021 | A | B | 2009 | 2021 | A | B | 2009 | 2021 | A | B | |
| No. of species | 111 | 116 | 95 | 87 | 76 | 86 | 67 | 41 | 72 | 46 | 34 | 27 | 54 | 48 | 38 | 30 |
| No. of individuals | 2348 | 1680 | 1259 | 977 | 1222 | 790 | 444 | 376 | 618 | 569 | 142 | 133 | 508 | 321 | 290 | 277 |
| NODF | 15.75 * | 10.63 * | 9.29 * | 9.40 * | 26.70 * | 19.62 * | 17.52 | 20.64 | 23.69 * | 18.18 | 13.89 | 15.12 | 19.70 | 14.02 | 14.23 | 17.49 |
| C | 0.11 | 0.07 | 0.07 | 0.07 | 0.20 | 0.14 | 0.14 | 0.17 | 0.18 | 0.14 | 0.11 | 0.12 | 0.15 | 0.11 | 0.11 | 0.14 |
| L/S | 3.66 | 2.30 | 2.05 | 2.00 | 2.66 | 1.87 | 1.77 | 1.93 | 2.32 | 1.59 | 1.16 | 1.17 | 1.90 | 1.40 | 1.28 | 1.44 |
| LD | 9.58 | 5.77 | 4.43 | 4.65 | 7.21 | 7.02 | 5.26 | 4.38 | 8.22 | 2.78 | 2.98 | 2.84 | 4.00 | 2.74 | 2.34 | 2.38 |
| IE | 0.63 | 0.53 | 0.50 | 0.54 | 0.64 | 0.60 | 0.63 | 0.63 | 0.68 | 0.42 | 0.55 | 0.54 | 0.54 | 0.43 | 0.40 | 0.40 |
| H2’ | 0.36 | 0.54 | 0.57 | 0.56 | 0.38 | 0.53 | 0.51 | 0.47 | 0.36 | 0.65 | 0.66 | 0.66 | 0.51 | 0.68 | 0.69 | 0.62 |
| V-ratio | 8.85 | 4.62 | 4.79 | 5.03 | 3.67 | 1.77 | 1.87 | 2.00 | 3.24 | 0.97 | 0.54 | 0.60 | 2.60 | 1.73 | 2.18 | 2.65 |
| RR | 0.90 | 0.84 | 0.81 | 0.80 | 0.93 | 0.91 | 0.89 | 0.87 | 0.92 | 0.80 | 0.70 | 0.70 | 0.87 | 0.84 | 0.80 | 0.79 |
| RM | 0.61 | 0.54 | 0.54 | 0.52 | 0.69 | 0.73 | 0.73 | 0.66 | 0.67 | 0.65 | 0.49 | 0.48 | 0.70 | 0.70 | 0.57 | 0.52 |
| RL | 0.99 | 0.97 | 0.96 | 0.95 | 0.99 | 0.97 | 0.98 | 0.95 | 0.98 | 0.89 | 0.85 | 0.84 | 0.97 | 0.97 | 0.94 | 0.91 |
| Park Scale | Deciduous | Riparian | Sclerophyllous | |
|---|---|---|---|---|
| βS | 0.758 | 0.691 | 0.667 | 0.681 |
| βWN | 0.163 | 0.225 | 0.237 | 0.338 |
| βST | −0.038 | −0.065 | −0.116 | −0.104 |
| βOS | 0.201 | 0.290 | 0.352 | 0.442 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinto, J.; Díaz-Castelazo, C.; Ramírez-Hernández, A.; Padilla, A.; Sánchez-Almodóvar, E.; Galante, E.; Micó, E. Interaction Networks Help to Infer the Vulnerability of the Saproxylic Beetle Communities That Inhabit Tree Hollows in Mediterranean Forests. Insects 2023, 14, 446. https://0-doi-org.brum.beds.ac.uk/10.3390/insects14050446
Quinto J, Díaz-Castelazo C, Ramírez-Hernández A, Padilla A, Sánchez-Almodóvar E, Galante E, Micó E. Interaction Networks Help to Infer the Vulnerability of the Saproxylic Beetle Communities That Inhabit Tree Hollows in Mediterranean Forests. Insects. 2023; 14(5):446. https://0-doi-org.brum.beds.ac.uk/10.3390/insects14050446
Chicago/Turabian StyleQuinto, Javier, Cecilia Díaz-Castelazo, Alfredo Ramírez-Hernández, Ascensión Padilla, Esther Sánchez-Almodóvar, Eduardo Galante, and Estefanía Micó. 2023. "Interaction Networks Help to Infer the Vulnerability of the Saproxylic Beetle Communities That Inhabit Tree Hollows in Mediterranean Forests" Insects 14, no. 5: 446. https://0-doi-org.brum.beds.ac.uk/10.3390/insects14050446