Sexual Behavior of Drosophila suzukii
Abstract
:1. Introduction
2. Experimental Section
2.1. Flies
2.2. Elements of Courtship and Mating Behavior
2.3. Diurnal Mating Activity
2.4. Age-Related Mating Activity and Reproduction
2.4.1. Age-Related Mating Activity
2.4.2. Age-Related Reproduction
2.5. Courtship Assay with Hexane Washed Females
2.6. Mating Assay with Antennectomized Males
2.7. Quantitative Analysis of Female Cuticular Hydrocarbons
2.8. Statistics
3. Results and Discussion
3.1. Description of Mating Behavior
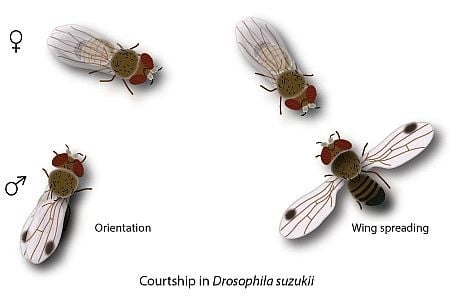
- Orientation: the male starts approaching the female and follows her if moving away. Fast male abdominal quivering and scissoring of the wings (see below) often accompany this initial behavior.
- Wing scissoring and flicking: when stopped, the male rapidly opens and closes both wings, sometimes with sharp flicking; wings do not fully close (semi-open) in comparison to the resting position (Figure 1a).
- Tapping: the male stretches a foreleg and strikes against the female abdomen, or middle or hind legs. Tapping results in either acceptance or rejection by the female, i.e., heed and stay or escape, respectively (Figure 1b).
- Wing spreading and abdominal quivering: the male now is in the visual field of the female. He orients to the front of the female and when stopped, quivers with the abdomen and displays wing scissoring keeping the wings spread at approximately 180° for several seconds (Figure 1c). From time to time, sharp flicking of wings occurs. During spreading, the wings are turned to a more vertical position exposing the upper side as well as the wing spot towards the female. In between repeated spreading, the wings are not fully closed.
- Foreleg rubbing: preening of legs is observed between various courtship elements.
- Circling: positioned in front of the female the male circles, wings open and facing the female, to the female’s right and left and back to the front (Figure 1c). When the male stops at the side of the female he extends both wings, or only the one directed to the female’s front (Figure 1d). After his initial focus on the female’s front the male later circles to her rear.
- Mounting: the male bends his abdomen down and forward, comes close to the female’s abdomen and thrusts under her wings. With the forelegs, the male grasps the female’s body and mounts upon for mating.

- Standstill: the female gradually stops moving and does not escape when the male is following and courting her.
- Genital spreading: the female spreads the ovipositor and moves the abdomen up and down when the male is courting.
- Ceased positioning: the female does not dip the abdomen downwards, rather holds it at resting position when the male approaches the abdomen.
- Lifting wings: the female shows a slight lifting of the wings that seems to facilitate the male’s mounting.
- Decamping: the female flies-off, runs or jumps away from the courting male.
- Kicking: depending upon the male’s position, the female kicks the male with front-, mid- or hind-legs to avoid his close approach.
- Spin: the female vigorously spins around when the male tries to mount her.
- Abdominal depression: the female dips her abdominal tip towards the substrate.
3.2. Diurnal Mating Activity

3.3. Age-Related Mating Activity and Reproduction

| Age [Days Post Emergence] When Females Were Checked for Off–Spring Production | Percentage of Replicates (n = 7) in Which Females Produce Offspring | |
|---|---|---|
| Freshly Emerged Females × Freshly Emerged Males | Freshly Emerged Females × 4-d-Old Males | |
| 0–2.0 | 0 | 0 |
| 2.5 | 43 | 100 |
| 3.0 | 57 | |
| 3.5 | 100 | |
3.4. Female-Derived Compounds as Possible Signals

3.5. Impact on Pest Management
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mendelson, T.C.; Shaw, K.L. The (mis)concept of species recognition. Trends Ecol. Evol. 2012, 27, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Hansson, B.S.; Nilsson, U.; Han, Q.; Sjöholm, M.; Skals, N.; Anton, S. Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Senses 2007, 32, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Ruther, J.; Thal, K.; Blaul, B.; Steiner, S. Behavioural switch in the sex pheromone response of Nasonia vitripennis females is linked to receptivity signalling. Animal Behav. 2010, 80, 1035–1040. [Google Scholar] [CrossRef]
- Palmer, C.R.; Kristan, W.B., Jr. Contextual modulation of behavioral choice. Curr. Opin. Neurobiol. 2011, 21, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Saveer, A.M.; Kromann, S.; Birgerson, G.; Bengtsson, M.; Lindblom, T.; Balkenius, A.; Hansson, B.S.; Witzgall, P.; Becher, P.G.; Ignell, R. Floral to green: Mating switches moth olfactory coding and preference. Proc. R. Soc. B 2012, 279, 2314–2322. [Google Scholar] [CrossRef] [PubMed]
- Kromann, S.H.; Saveer, A.M.; Binyameen, M.; Bengtsson, M.; Birgersson, G.; Hansson, B.S.; Schlyter, F.; Witzgall, P.; Ignell, R.; Becher, P.G. Concurrent modulation of neuronal and behavioural olfactory responses to sex and host plant cues in a male moth. Proc. R. Soc. B 2015, 282, 20141884. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Pheromones and Animal Behaviour: Communication by Smell and Taste; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Smadja, C.; Butlin, R.K. On the scent of speciation: The chemosen-sory system and its role in premating isolation. Heredity 2009, 102, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Kaissling, K.-E. Pheromone reception in insects: The example of silk moths. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 99–146. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2014. Available online: http://www.pherobase.com (accessed on 20 January 2015).
- Benton, R. Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci. 2007, 30, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, H.J.; Goodwin, S.F. Courtship behavior in Drosophila melanogaster: Towards a “courtship connectome”. Curr. Opin. Neurobiol. 2013, 23, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, S.; Grabe, V.; Omondi, A.B.; Ignell, R.; Becher, P.G.; Hansson, B.S.; Sachse, S.; Witzgall, P. Love makes smell blind: Mating suppresses pheromone attraction in Drosophila females via OR65a olfactory neurons. Sci. Rep. 2014, 4, 7119. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.C.; Oriatti, C.I.; Anfora, G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull. Insectol. 2012, 65, 149–160. [Google Scholar]
- Mazzoni, V.; Anfora, G.; Virant-Doberlet, M. Substrate vibrations during courtship in three Drosophila species. PLOS ONE 2013, 8, e80708. [Google Scholar] [CrossRef] [PubMed]
- Dekker, T.; Revadi, S.; Mansourian, S.; Ramasamby, S.; Lebreton, S.; Becher, P.G.; Angeli, S.; Rota-Stabelli, O.; Anfora, G. Loss of Drosophila pheromone reverses its role in sexual communication in Drosophila suzukii. Proc. R. Soc. B 2015. [Google Scholar] [CrossRef]
- Spieth, H.T. Courtship behavior in Drosophila. Annu. Rev. Entomol. 1974, 19, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Farine, J.-P.; Ferveur, J.-F.; Everaerts, C. Volatile Drosophila cuticular pheromones are affected by social but not sexual experience. PLOS ONE 2012, 7, e40396. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.; Ferveur, J.-F. Evolution and genetic control of mate recognition and stimulation in Drosophila. Behav. Process. 1996, 35, 35–54. [Google Scholar] [CrossRef]
- Cobb, M.; Burnet, B.; Blizard, R.; Jallon, J.-M. Courtship in Drosophila sechellia: It’s structure, functional aspects, and relationship to those of other members of the Drosophila melanogaster species subgroup. J. Insect. Behav. 1989, 2, 63–89. [Google Scholar] [CrossRef]
- Markow, T.A. Female remating, operational sex ratio, and the arena of sexual selection in Drosophila species. Evolution 2002, 56, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Laturney, M.; Billeter, J.C. Neurogenetics of female reproductive behaviors in Drosophila melanogaster. Adv. Genet. 2013, 85, 1–108. [Google Scholar]
- Markow, T.A. Courtship behavior and control of reproductive isolation between Drosophila mojavensis and Drosophila arizonensis. Evolution 1981, 35, 1022–1026. [Google Scholar] [CrossRef]
- Marcillac, F.; Houot, B.; Ferveur, J.-F. Revisited roles of Drosophila female pheromones. Chem. Senses 2005, 30, i273–i274. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, A.; Widmer, A.; Dickson, B.J. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature 2007, 446, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Ishida, N. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc. Natl. Acad. Sci. USA 2001, 98, 9221–9225. [Google Scholar] [CrossRef] [PubMed]
- Tauber, E.; Roe, H.; Costa, R.; Hennessy, J.M.; Kyriacou, C.P. Temporal mating isolation driven by a behavioral gene in Drosophila. Curr. Biol. 2003, 13, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Krishnan, P.; Hardin, P.; Amrein, H. Nocturnal male sex drive in Drosophila. Curr. Biol. 2007, 17, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Kwok, R.S.; Zalom, F.G.; Chiu, J.C. Integrating circadian activity and gene expression profiles to predict chronotoxicity of Drosophila suzukii response to insecticides. PLOS ONE 2013, 8, e68472. [Google Scholar] [CrossRef] [PubMed]
- Markow, T.A.; O’Grady, P.M. Reproductive ecology of Drosophila. Func. Ecol. 2008, 22, 747–759. [Google Scholar] [CrossRef]
- Kanzawa, T. Studies on Drosophila suzukii Mats. J. Plant Protect. 1936, 23, 66–70. [Google Scholar]
- Kambysellis, M.P.; Craddock, E.M. Insemination patterns in Hawaiian Drosophila species (Diptera: Drosophilidae) correlate with ovarian development. J. Insect Behav. 1991, 4, 83–100. [Google Scholar] [CrossRef]
- Markow, T.A. Forced matings in natural populations of Drosophila. Am. Nat. 2000, 156, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Jallon, J.M.; David, J.R. Variations in cuticular hydrocarbons among the eight species of the Drosophila melanogaster subgroup. Evolution 1987, 4, 294–302. [Google Scholar] [CrossRef]
- Ferveur, J.-F. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav. Genet. 2005, 35, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Bontonou, G.; Wicker-Thomas, C. Sexual communication in the Drosophila genus. Insects 2014, 5, 439–458. [Google Scholar] [CrossRef]
- Jallon, J.M. A few chemical words exchanged by Drosophila during courtship and mating. Behav. Genet. 1984, 14, 441–478. [Google Scholar] [CrossRef] [PubMed]
- Antony, C.; Davis, T.L.; Carlson, D.A.; Pechine, J.-M.; Jallon, J.M. Compared behavioural responses of male Drosophila melanogaster (Canton S) to natural and synthetic aphrodisiacs. J. Chem. Ecol. 1985, 11, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Ferveur, J.-F.; Savarit, F.; O’Kane, C.-J.; Sureau, G.; Greenspan, R.J.; Jallon, J.-M. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science 1997, 276, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Billeter, J.C.; Atallah, J.; Krupp, J.J.; Millar, J.G.; Levine, J.D. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature 2009, 461, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Thistle, R.; Cameron, P.; Ghorayshi, A.; Dennison, L.; Scott, K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell 2012, 149, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Antony, C.; Jallon, J.M. Evolution des hydrocarbures comportementalement actifs des Drosophila melanogaster au cours de la maturation sexuelle. C. R. Acad. Sci. Paris Ser. D 1981, 292, 239–242. [Google Scholar]
- Everaerts, C.; Lacaille, F.; Ferveur, J.-F. Is mate choice in Drosophila males guided by olfactory or gustatory pheromones? Animal Behav. 2010, 79, 1135–1146. [Google Scholar] [CrossRef]
- Grillet, M.; Dartevelle, L.; Ferveur, J.-F. A Drosophila male pheromone affects female sexual receptivity. Proc. R. Soc. B 2006, 273, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Wiman, N.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLOS ONE 2014, 9, e106909. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revadi, S.; Lebreton, S.; Witzgall, P.; Anfora, G.; Dekker, T.; Becher, P.G. Sexual Behavior of Drosophila suzukii. Insects 2015, 6, 183-196. https://0-doi-org.brum.beds.ac.uk/10.3390/insects6010183
Revadi S, Lebreton S, Witzgall P, Anfora G, Dekker T, Becher PG. Sexual Behavior of Drosophila suzukii. Insects. 2015; 6(1):183-196. https://0-doi-org.brum.beds.ac.uk/10.3390/insects6010183
Chicago/Turabian StyleRevadi, Santosh, Sébastien Lebreton, Peter Witzgall, Gianfranco Anfora, Teun Dekker, and Paul G. Becher. 2015. "Sexual Behavior of Drosophila suzukii" Insects 6, no. 1: 183-196. https://0-doi-org.brum.beds.ac.uk/10.3390/insects6010183