Non- and Quasi-Equilibrium Multi-Phase Field Methods Coupled with CALPHAD Database for Rapid-Solidification Microstructural Evolution in Laser Powder Bed Additive Manufacturing Condition
Abstract
:1. Introduction
2. Model Description and Computational Procedure
2.1. Non- and Quasi-Equilibrium Multi-Phase Field Method
2.2. Sublattice Model of γ in the CALPHAD Framework
2.3. Computational Methods and Common Conditions
2.4. Permeability Value
3. Equiaxed Microstructure Evolution
3.1. Specific Model Conditions for Equiaxed Microstructure Evolution
3.2. Results and Discussion
4. Columnar Microstructure Evolution
4.1. Specific Model Conditions for Columnar Microstructure Evolution
4.2. Experimental Conditions
4.3. Results and Discussion
5. Conclusions
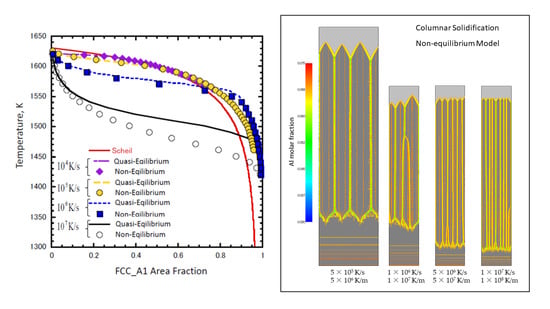
- The temperature-γ fraction relationships under a cooling rate of 105 K/s for non- and quasi-equilibrium MPFMs in the two-dimensional equiaxed simulations were in good agreement with each other. They were quite close to the Scheil model profile at 104 K/s.
- The differences between non- and quasi-equilibrium methods grew with the cooling rate. The non-equilibrium solidification tendency was strengthened with the cooling rate of 106 K/s.
- Columnar solidification microstructure evolutions were performed in cooling rates from 5 × 105 K/s to 1 × 107 K/s at various temperature gradient values while maintaining a constant interface velocity of 0.1 m/s. The results showed that, as the cooling rate increased, the cell space decreased in both equilibrium methods. The average cell space in the non-equilibrium method was larger than that in the quasi-equilibrium method with each cooling rate.
- The thermal undercooling of the non-equilibrium method was much larger than that of the quasi-equilibrium method, whereas the diffusional undercooling was almost the same for both.
- The non-equilibrium MPFM provides us with a more accurate tool for solidification microstructure estimation in LPBF.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
General Representation of the Chemical Potential for the FCC_L12 Phase
Appendix B
Additional Calculation Using Twice the Width, 5 μm, for Non-Equilibrium MPFM unde the Condition of Case (a)

References
- Gorsse, S.; Hutchinson, C.; Gouné, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [Green Version]
- Yan, F.; Xiong, W.; Faierson, E.J. Grain structure control of additively manufactured metallic materials. Materials 2017, 10, 1260. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.C.; Brice, D.A.; Samimi, P.; Ghamarian, I.; Fraser, H.L. Microstructural control of additively manufactured metallic materials. Annu. Rev. Mater. Res. 2016, 46, 63–91. [Google Scholar] [CrossRef]
- Kok, Y.; Tan, X.P.; Wang, P.; Nai, M.L.S.; Loh, N.H.; Liu, E.; Tor, S.B. Anisotropy and heterogeneity of microstructure and mechanical properties in metal additive manufacturing: A critical review. Mater. Des. 2018, 139, 565–586. [Google Scholar] [CrossRef]
- Thampy, V.; Fong, A.F.; Calta, N.P.; Wang, J.; Martin, A.A.; Depend, P.J.; Kiss, A.M.; Guss, G.; Xing, Q.; Ott, R.T.; et al. Subsurface cooling rates and microstructural response during laser based metal additive manufacturing. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Farshidianfar, M.H.; Khajepour, A.; Gerlich, A.P. Effect of real-time cooling rate on microstructure in Laser Additive Manufacturing. J. Mater. Process. Technol. 2016, 231, 468–478. [Google Scholar] [CrossRef]
- Scipioni Bertoli, U.S.; Guss, G.; Wu, S.; Matthews, M.J.; Schoenung, J.M. In-situ characterization of laser-powder interaction and cooling rates through high-speed imaging of powder bed fusion additive manufacturing. Mater. Des. 2017, 135, 385–396. [Google Scholar] [CrossRef]
- Promoppatum, P.; Yao, S.-C.; Pistorius, P.C.; Rollett, A.D.; Coutts, P.J.; Lia, F.; Martukanitz, R. Numerical modeling and experimental validation of thermal history and microstructure for additive manufacturing of an Inconel 718 product. Prog. Addit. Manuf. 2018, 3, 15–32. [Google Scholar] [CrossRef]
- Shimono, Y.; Oba, M.; Nomoto, S. Solidification simulation of direct energy deposition process by multi-phase field method coupled with thermal analysis. Model. Simul. Mater. Sci. Eng. 2019, 27, 074006. [Google Scholar] [CrossRef] [Green Version]
- Karayagiz, K.; Elwany, A.; Tapia, G.; Franco, B.; Johnson, L.; Ma, J.; Karaman, I.; Arróyave, R. Numerical and experimental analysis of heat distribution in the laser powder bed fusion of Ti-6Al-4V IISE. Transactions 2019, 51, 136–152. [Google Scholar] [CrossRef]
- Watari, N.; Ogura, Y.; Yamazaki, N.; Inoue, Y.; Kamitani, K.; Fujiya, Y.; Toyoda, M.; Goya, S.; Watanabe, T. Two-fluid model to simulate metal powder bed fusion additive manufacturing. J. Fluid Sci. Technol. 2018, 13, JFST0010. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chew, Y.; Bi, G.; Yao, X.; Zhang, B.; Bai, J.; Moon, S.K. Numerical and experimental study of laser aided additive manufacturing for melt-pool profile and grain orientation analysis. Mater. Des. 2018, 137, 286–297. [Google Scholar] [CrossRef]
- Rolchigo, M.R.; Mendoza, M.Y.; Samimi, P.; Brice, D.A.; Martin, B.; Collins, P.C.; LeSar, R. Modeling of ti-W solidification microstructures under additive manufacturing conditions. Metall. Mater. Trans. A 2017, 48, 3606–3622. [Google Scholar] [CrossRef] [Green Version]
- Lian, Y.; Gan, Z.; Yu, C.; Kats, D.; Liu, W.K.; Wagner, G.J. A cellular automaton finite volume method for microstructure evolution during additive manufacturing. Mater. Des. 2019, 169. [Google Scholar] [CrossRef]
- Ghosh, S.; McReynolds, K.; Guyer, J.E.; Banerjee, D. Simulation of temperature, stress and microstructure fields during laser deposition of Ti–6Al–4V. Model. Simul. Mater. Sci. Eng. 2018, 26, 075005. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, P.W.; Ji, Y.; Liu, Y.; Horstemeyer, M.H.; Chen, L. Investigation on microsegregation of IN718 alloy during additive manufacturing via integrated phase-field and finite-element modeling. J. Mater. Eng. Perform. 2018, 28, 657–665. [Google Scholar] [CrossRef]
- Yang, Y.; Kühn, P.; Yi, M.; Egger, H.; Xu, B.-X. Non-isothermal phase-field modeling of heat–melt–microstructure-coupled processes during powder bed fusion. JOM 2020, 72, 1719–1733. [Google Scholar] [CrossRef]
- Acharya, R.; Sharon, J.A.; Staroselsky, A. Prediction of microstructure in laser powder bed fusion process. Acta Mater. 2017, 124, 360–371. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J. Phase field simulation of dendritic solidification of Ti-6Al-4V during additive manufacturing process. JOM 2018, 70, 2392–2399. [Google Scholar] [CrossRef] [Green Version]
- Boussinot, G.; Apel, M.; Zielinski, J.; Hecht, U.; Schleifenbaum, J.H. Strongly out-of-equilibrium columnar solidification during the Laser Powder-Bed Fusion additive manufacturing process. Phys. Rev. Appl. 2019, 11, 014025. [Google Scholar] [CrossRef] [Green Version]
- Kitano, H.; Tsuji, M.; Kusano, M.; Yumoto, A.; Watanabe, M. Effect of plastic strain on the solidification cracking of Hastelloy-X in the selective laser melting process. Addit. Manuf. 2021, 37, 101742. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Xu, L.; Xu, J.; Ren, X.; Chen, X. Cracking mechanism and susceptibility of laser melting deposited Inconel 738 superalloy. Mater. Des. 2019, 183, 108105. [Google Scholar] [CrossRef]
- Cloots, M.; Uggowitzer, P.J.; Wegener, K. Investigations on the microstructure and crack formation of IN738LC samples processed by selective laser melting using Gaussian and doughnut profiles. Mater. Des. 2016, 89, 770–784. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Dinda, G.P. Direct laser metal deposition of Inconel 738. Mater. Sci. Eng. A 2019, 740–741, 1–13. [Google Scholar] [CrossRef]
- Steinbach, I.; Zhang, L.; Plapp, M. Phase-field model with finite interface dissipation. Acta Mater. 2012, 60, 2689–2701. [Google Scholar] [CrossRef]
- Zhang, L.; Steinbach, I. Phase-field model with finite interface dissipation: Extension to multi-component multi-phase alloys. Acta Mater. 2012, 60, 2702–2710. [Google Scholar] [CrossRef]
- Karayagiz, K.; Johnson, L.; Seede, R.; Attari, V.; Zhang, B.; Huang, X.; Ghosh, S.; Duong, T.; Karaman, I.; Elwany, A.; et al. Finite interface dissipation phase field modeling of Ni–Nb under additive manufacturing conditions. Acta Mater. 2020, 185, 320–339. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.G.; Kim, W.T.; Suzuki, T. Phase-field model for binary alloys. Phys. Rev. Estat. Phys. Plasmasfluidsand Relat. Interdiscip. Top. 1999, 60, 7186–7197. [Google Scholar] [CrossRef]
- Zhang, L.; Stratmann, M.; Du, Y.; Sundman, B.; Steinbach, I. Incorporating the CALPHAD sublattice approach of ordering into the phase-field model with finite interface dissipation. Acta Mater. 2015, 88, 156–169. [Google Scholar] [CrossRef]
- Steinbach, I. Phase-field models in materials science. Model. Simul. Mater. Sci. Eng. 2009, 17, 073001. [Google Scholar] [CrossRef]
- Nomoto, S.; Wakameda, H.; Segawa, M.; Yamanaka, A.; Koyama, T. Solidification analysis by non-equilibrium phase field model using thermodynamics data estimated by machine learning. Model. Simul. Mater. Sci. Eng. 2019, 27, 084008. [Google Scholar] [CrossRef]
- Kobayashi, R. Modeling and numerical simulations of dendritic crystal growth. Phys. D 1993, 63, 410–423. [Google Scholar] [CrossRef]
- Thermodynamics and Properties Software. Available online: https://thermocalc.com/products/thermo-calc/ (accessed on 30 March 2021).
- Nickel-Based Superalloys Databases. Available online: https://thermocalc.com/products/databases/nickel-based-alloys/ (accessed on 30 March 2021).
- Short for Thermodynamic Calculation Interface. Available online: https://thermocalc.com/products/software-development-kits/#TQ-Interface (accessed on 30 March 2021).
- Prasad, A.; Yuan, L.; Lee, P.; Patel, M.; Qiu, D.; Easton, M.; StJohn, D. Towards understanding grain nucleation under Additive Manufacturing solidification conditions. Acta Mater. 2020, 195, 392–403. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Appendix 6 Thermodynamics of rapid solidification. In Fundamentals of Solidification, 4th ed.; Trans Tech Publications Ltd: Zürich, Switzerland, 1998; pp. 220–225. [Google Scholar]
- Segawa, M.; Yamanaka, A.; Nomoto, S. Multi-phase-field simulation of cyclic phase transformation in Fe-C-Mn and Fe-C-Mn-Si alloys. Comput. Mater. Sci. 2017, 136, 67–75. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Chapter four: Solidification microstructure: Cells and dendrite. In Fundamentals of Solidification, 4th ed.; Trans Tech Publications Ltd: Zürich, Switzerland, 1998; pp. 63–88. [Google Scholar]
- Kusano, M.; Kitano, H.; Watanabe, M. Novel calibration strategy for validation of finite element thermal analysis of selective laser melting process using Bayesian optimization. Addit. Manuf. reviewed.













| Case | Cooling Rate, R (K/s) | Temperature Gradient, G (K/m) |
|---|---|---|
| (a) | 5 × 105 | 5 × 106 |
| (b) | 1 × 106 | 1 × 107 |
| (c) | 5 × 106 | 5 × 107 |
| (d) | 1 × 107 | 1 × 108 |
| Case | Non-Equilibrium MPFM | Quasi-Equilibrium MPFM |
|---|---|---|
| (a) | 1.43 (1) | 0.45 (1) |
| (b) | 0.89 (0.62) | 0.36 (0.8) |
| (c) | 0.45 (0.32) | 0.29 (0.64) |
| (d) | 0.31 (0.22) | 0.16 (0.35) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomoto, S.; Segawa, M.; Watanabe, M. Non- and Quasi-Equilibrium Multi-Phase Field Methods Coupled with CALPHAD Database for Rapid-Solidification Microstructural Evolution in Laser Powder Bed Additive Manufacturing Condition. Metals 2021, 11, 626. https://0-doi-org.brum.beds.ac.uk/10.3390/met11040626
Nomoto S, Segawa M, Watanabe M. Non- and Quasi-Equilibrium Multi-Phase Field Methods Coupled with CALPHAD Database for Rapid-Solidification Microstructural Evolution in Laser Powder Bed Additive Manufacturing Condition. Metals. 2021; 11(4):626. https://0-doi-org.brum.beds.ac.uk/10.3390/met11040626
Chicago/Turabian StyleNomoto, Sukeharu, Masahito Segawa, and Makoto Watanabe. 2021. "Non- and Quasi-Equilibrium Multi-Phase Field Methods Coupled with CALPHAD Database for Rapid-Solidification Microstructural Evolution in Laser Powder Bed Additive Manufacturing Condition" Metals 11, no. 4: 626. https://0-doi-org.brum.beds.ac.uk/10.3390/met11040626