Molecular Survey of Bartonella Species in Stray Cats and Dogs, Humans, and Questing Ticks from Portugal
Abstract
:1. Introduction
2. Results
2.1. Tick Samples and Molecular Detection of Bartonella spp.
2.2. Detection of Bartonella spp. in Human Blood and Blood from Non-Domiciliated Cats and Dogs
3. Discussion
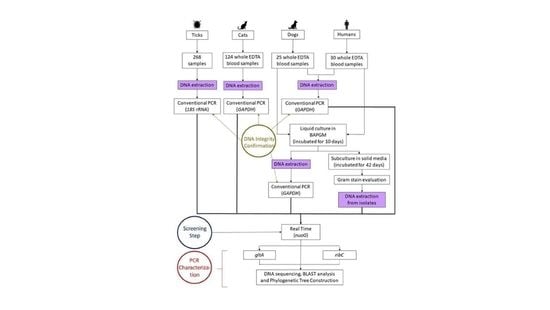
4. Material and Methods
4.1. Ethics Statement
4.2. Biological Samples Collection
4.2.1. Cats and Dogs Whole Blood Sampling
4.2.2. Humans Whole Blood Sampling
4.2.3. Ticks
4.3. Cultivation of Bartonella from Dog and Human Blood Samples
4.4. DNA Extraction
4.5. Polymerase Chain Reaction (PCR)
4.5.1. PCR Inhibitors and DNA Integrity
4.5.2. Molecular Detection and Characterization of Bartonella spp.
4.5.3. Sequences and Phylogenetic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breitschwerdt, E.B.; Maggi, R.G.; Chomel, B.B.; Lappin, M.R. Bartonellosis: An Emerging Infectious Disease of Zoonotic Importance to Animals and Human Beings. J. Vet. Emerg. Crit. Care 2010, 20, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Boulouis, H.J.; Breitschwerdt, E.B.; Kasten, R.W.; Vayssier-Taussat, M.; Birtles, R.J.; Koehler, J.E.; Dehio, C. Ecological Fitness and Strategies of Adaptation of Bartonella Species to Their Hosts and Vectors. Vet. Res. 2009, 40, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella Species, an Emerging Cause of Blood-Culture-Negative Endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regier, Y.; Órourke, F.; Kempf, V.A.J. Bartonella spp.—A Chance to Establish One Health Concepts in Veterinary and Human Medicine. Parasites Vectors 2016, 9, 261, Erratum in Parasites Vectors 2016, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Billeter, S.A.; Levy, M.G.; Chomel, B.B.; Breitschwerdt, E.B. Vector Transmission of Bartonella Species with Emphasis on the Potential for Tick Transmission. Med. Vet. Entomol. 2008, 22, 1–15. [Google Scholar] [CrossRef]
- Abbott, R.C.; Chomel, B.B.; Kasten, R.W.; Floyd-Hawkins, K.A.; Kikuchi, Y.; Koehler, J.E.; Pedersen, N.C. Experimental and Natural Infection with Bartonella henselae in Domestic Cats. Comp. Immunol. Microbiol. Infect. Dis. 1997, 20, 41–51. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, C.M.; Chang, C.C. Unknown Fever and Back Pain Caused by Bartonella henselae in a Veterinarian after a Needle Puncture: A Case Report and Literature Review. Vector-Borne Zoonotic Dis. 2011, 11, 589–591. [Google Scholar] [CrossRef]
- Silva, M.N.; Vieira-Damiani, G.; Ericson, M.E.; Gupta, K.; Gilioli, R.; De Almeida, A.R.; Drummond, M.R.; Lania, B.G.; De Almeida Lins, K.; Soares, T.C.B.; et al. Bartonella henselae Transmission by Blood Transfusion in Mice. Transfusion 2016, 56, 1556–1559. [Google Scholar] [CrossRef] [Green Version]
- Pitassi, L.H.U.; Cintra, M.L.; Ferreira, M.R.M.; Magalhes, R.F.; Ferreira Velho, P.E.N. Blood Cell Findings Resembling Bartonella spp. Ultrastruct. Pathol. 2010, 34, 2–6. [Google Scholar] [CrossRef]
- Boulouis, H.J.; Chang, C.C.; Henn, J.B.; Kasten, R.W.; Chomel, B.B. Factors Associated with the Rapid Emergence of Zoonotic Bartonella Infections. Vet. Res. 2005, 36, 383–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chomel, B.B.; Boulouis, H.J.; Breitschwerdt, E.B. Cat Scratch Disease and Other Zoonotic Bartonella Infections. J. Am. Vet. Med. Assoc. 2004, 224, 1270–1279. [Google Scholar] [CrossRef] [Green Version]
- Dehio, C. Molecular and Cellular Basis of Bartonella Pathogenesis. Annu. Rev. Microbiol. 2004, 58, 365–390. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Billeter, S.A.; Breitschwerdt, E.B.; Chomel, B.B.; Raoult, D. Potential for Tick-Borne Bartonelloses. Emerg. Infect. Dis. 2010, 16, 385–391. [Google Scholar] [CrossRef] [PubMed]
- King, L.J.; Anderson, L.R.; Blackmore, C.G.; Blackwell, M.J.; Lautner, E.A.; Marcus, L.C.; Meyer, T.E.; Monath, T.P.; Davis, R.M.; Glasser, J.H.; et al. Executive Summary of the AVMA One Health Initiative Task Force Report Preface and Acknowledgments. J. Am. Vet. Med. Assoc. 2008, 233, 259–261. [Google Scholar] [CrossRef]
- Álvarez-Fernández, A.; Breitschwerdt, E.B.; Solano-Gallego, L. Bartonella Infections in Cats and Dogs Including Zoonotic Aspects. Parasites Vectors 2018, 11, 624. [Google Scholar] [CrossRef]
- Childs, J.E.; Olson, J.G.; Wolf, A.; Cohen, N.; Fakile, Y.; Rooney, J.A.; Bacellar, F.; Regnery, R.L. Prevalence of Antibodies to Rochalimaea Species (Cat-Scratch Disease Agent) in Cats. Vet. Rec. 1995, 136, 519–520. [Google Scholar] [CrossRef]
- Maia, C.; Almeida, B.; Coimbra, M.; Fernandes, M.C.; Cristóvão, J.M.; Ramos, C.; Martins, Â.; Martinho, F.; Silva, P.; Neves, N.; et al. Bacterial and Protozoal Agents of Canine Vector-Borne Diseases in the Blood of Domestic and Stray Dogs from Southern Portugal. Parasites Vectors 2015, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Persichetti, M.F.; Pennisi, M.G.; Vullo, A.; Masucci, M.; Migliazzo, A.; Solano-Gallego, L. Clinical Evaluation of Outdoor Cats Exposed to Ectoparasites and Associated Risk for Vector-Borne Infections in Southern Italy. Parasites Vectors 2018, 11, 136. [Google Scholar] [CrossRef]
- de Andrade Oliveira Cruz, T.N.; Gonçalves, L.R.; Furquim, M.E.C.; André, M.R.; Munhoz, A.D.; Carlos, R.S.A.; Silva, F.L. Threat under Cats’ Claws: Molecular Detection and Risk Factors for Zoonotic Bartonella Species in Blood and Claw Samples from Cats in Brazil. Acta Trop. 2022, 232, 106496. [Google Scholar] [CrossRef]
- Chomel, B.B.; Kasten, R.W. Bartonellosis, an Increasingly Recognized Zoonosis. J. Appl. Microbiol. 2010, 109, 743–750. [Google Scholar] [CrossRef]
- Furquim, M.E.C.; do Amaral, R.; Dias, C.M.; Gonçalves, L.R.; Perles, L.; de Paula Lima, C.A.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Genetic Diversity and Multilocus Sequence Typing Analysis of Bartonella Henselae in Domestic Cats from Southeastern Brazil. Acta Trop. 2021, 222, 106037. [Google Scholar] [CrossRef]
- de Fátima Gonçalves Lopez Silva, M.; Mesquita, F.A.S.; da Costa Neves, B.M.; de Oliveira, S.V. Epidemiological Notes about Bartonellosis Reactive Samples in Brazil. J. Infect. Dis. Epidemiol. 2019, 5, 081. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Walker, R.; Bittencourt, P.; MacHado, R.Z.; Benevenute, J.L.; Do Amaral, R.B.; Gonçalves, L.R.; André, M.R. Prevalence, Hematological Findings and Genetic Diversity of Bartonella spp. in Domestic Cats from Valdivia, Southern Chile. Parasitology 2017, 144, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Duscher, G.G.; Hodzić, A.; Potkonjak, A.; Leschnik, M.W.; Spergser, J. Bartonella henselae and Rickettsia felis Detected in Cat Fleas (Ctenocephalides Felis) Derived from Eastern Austrian Cats. Vector-Borne Zoonotic Dis. 2018, 18, 282–284. [Google Scholar] [CrossRef]
- Dillon, B.; Valenzuela, J.; Don, R.; Blanckenberg, D.; Wigney, D.I.; Malik, R.; Morris, A.J.; Robson, J.M.; Iredell, J. Limited Diversity among Human Isolates of Bartonella henselae. J. Clin. Microbiol. 2002, 40, 4691–4699. [Google Scholar] [CrossRef] [Green Version]
- Srisanyong, W.; Takhampunya, R.; Boonmars, T.; Kerdsin, A.; Suksawat, F. Prevalence of Bartonella henselae, Bartonella clarridgeiae, and Bartonella vinsonii Subsp. Berkhoffii in Pet Cats from Four Provincial Communities in Thailand. Thai J. Vet. Med. 2016, 46, 663–670. [Google Scholar]
- Inoue, K.; Kabeya, H.; Hagiya, K.; Kosoy, M.Y.; Une, Y.; Yoshikawa, Y.; Maruyama, S. Multi-Locus Sequence Analysis Reveals Host Specific Association between Bartonella washoensis and Squirrels. Vet. Microbiol. 2011, 148, 60–65. [Google Scholar] [CrossRef]
- Ansil, B.R.; Mendenhall, I.H.; Ramakrishnan, U. High Prevalence and Diversity of Bartonella in Small Mammals from the Biodiverse Western Ghats. PLoS Negl. Trop. Dis. 2021, 15, e0009178. [Google Scholar] [CrossRef]
- Calchi, A.C.; Vultão, J.G.; Alves, M.H.; Yogui, D.R.; Desbiez, A.L.J.; Amaral, R.B.; Santi, M.; Teixeira, M.M.G.; Werther, K.; Machado, R.Z.; et al. Multi-Locus Sequencing Reveals a Novel Bartonella in Mammals from the Superorder Xenarthra. Transbound. Emerg. Dis. 2020, 67, 2020–2033. [Google Scholar] [CrossRef]
- Oteo, J.A.; Maggi, R.; Portillo, A.; Bradley, J.; García-Álvarez, L.; San-Martín, M.; Roura, X.; Breitschwerdt, E. Prevalence of Bartonella spp. by Culture, PCR and Serology, in Veterinary Personnel from Spain. Parasites Vectors 2017, 10, 553. [Google Scholar] [CrossRef] [Green Version]
- Chomel, B.B.; Boulouis, H.J.; Maruyama, S.; Breitschwerdt, E.B. Bartonella spp. in Pets and Effect on Human Health. Emerg. Infect. Dis. 2006, 12, 389–394. [Google Scholar] [CrossRef]
- Lashnits, E.; Neupane, P.; Bradley, J.M.; Richardson, T.; Maggi, R.G.; Breitschwerdt, E.B. Comparison of Serological and Molecular Assays for Bartonella Species in Dogs with Hemangiosarcoma. Pathogens 2021, 10, 794. [Google Scholar] [CrossRef]
- Drummond, M.R.; Dos Santos, L.S.; Silva, M.N.D.; de Almeida, A.R.; De Paiva Diniz, P.P.V.; Angerami, R.; Velho, P.E.N.F. False Negative Results in Bartonellosis Diagnosis. Vector-Borne Zoonotic Dis. 2019, 19, 453–454. [Google Scholar] [CrossRef] [Green Version]
- Pérez, C.; Maggi, R.G.; Diniz, P.P.V.P.; Breitschwerdt, E.B. Molecular and Serological Diagnosis of Bartonella Infection in 61 Dogs from the United States. J. Vet. Intern. Med. 2011, 25, 805–810. [Google Scholar] [CrossRef]
- Roura, X.; Santamarina, G.; Tabar, M.D.; Francino, O.; Altet, L. Polymerase Chain Reaction Detection of Bartonella spp. in Dogs from Spain with Blood Culture-Negative Infectious Endocarditis. J. Vet. Cardiol. 2018, 20, 267–275. [Google Scholar] [CrossRef]
- Mosbacher, M.E.; Klotz, S.; Klotz, J.; Pinnas, J.L. Bartonella henselae and the Potential for Arthropod Vector-Borne Transmission. Vector-Borne Zoonotic Dis. 2011, 11, 471–477. [Google Scholar] [CrossRef]
- Tobar, B.Z.; Lapsley, W.D.; Swain, W.L.; Jaffe, D.A.; Setien, A.A.; Galvez-Romero, G.; Obregon-Morales, C.; Olave-Leyva, J.I.; Chomel, B.B. Bartonella in Dogs and Fleas from Tulancingo, Hidalgo, Mexico. Med. Vet. Entomol. 2020, 34, 302–308. [Google Scholar] [CrossRef]
- Müller, A.; Soto, F.; Sepúlveda, M.; Bittencourt, P.; Benevenute, J.L.; Ikeda, P.; Machado, R.Z.; André, M.R. Bartonella vinsonii Subsp. berkhoffii and B. henselae in Dogs. Epidemiol. Infect. 2018, 146, 1202–1204. [Google Scholar] [CrossRef] [Green Version]
- André, M.R.; Canola, R.A.M.; Braz, J.B.; Perossi, I.F.S.; Calchi, A.C.; Ikeda, P.; Machado, R.Z.; de Oliveira Vasconcelos, R.; Camacho, A.A. Aortic Valve Endocarditis Due to Bartonella clarridgeiae in a Dog in Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 661–670. [Google Scholar] [CrossRef]
- Maurin, M.; Raoult, D. Bartonella (rochalimaea) quintana Infections. Clin. Microbiol. Rev. 1996, 9, 273–292. [Google Scholar] [CrossRef]
- Koehler, J.E.; Sanchez, M.A.; Tye, S.; Garrido-Rowland, C.S.; Chen, F.M.; Maurer, T.; Cooper, J.L.; Olson, J.G.; Reingold, A.L.; Hadley, W.K.; et al. Prevalence of Bartonella Infection among Human Immunodeficiency Virus-Infected Patients with Fever. Clin. Infect. Dis. 2003, 37, 559–566. [Google Scholar] [CrossRef]
- Fournier, P.E.; Lelievre, H.; Eykyn, S.J.; Mainardi, J.L.; Marrie, T.J.; Bruneel, F.; Roure, C.; Nash, J.; Clave, D.; James, E.; et al. Epidemiologic and Clinical Characteristics of Bartonella quintana and Bartonella henselae Endocarditis: A Study of 48 Patients. Medicine 2001, 80, 245–251. [Google Scholar] [CrossRef]
- Lantos, P.M.; Maggi, R.G.; Ferguson, B.; Varkey, J.; Park, L.P.; Breitschwerdt, E.B.; Woods, C.W. Detection of Bartonella Species in the Blood of Veterinarians and Veterinary Technicians: A Newly Recognized Occupational Hazard? Vector-Borne Zoonotic Dis. 2014, 14, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Edvinsson, M.; Norlander, C.; Nilsson, K.; Mårtensson, A.; Skoog, E.; Olsen, B. Bartonella spp. Seroprevalence in Tick-Exposed Swedish Patients with Persistent Symptoms. Parasites Vectors 2021, 14, 530. [Google Scholar] [CrossRef]
- Łysakowska, M.E.; Brzezińska, O.; Szybka, M.; Konieczka, M.; Moskwa, S.; Brauncajs, M.; Makowska, J.; Pastuszak-Lewandoska, D.; Grzegorczyk, J. The Seroprevalence of Bartonella spp. in the Blood of Patients with Musculoskeletal Complaints and Blood Donors, Poland: A Pilot Study. Clin. Rheumatol. 2019, 38, 2691–2698. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Luz, T.; Santos, A.S.; De Sousa, R.; Lopes de Carvalho, I.; Zé-Zé, L.; Amaro, F.; Parreira, P.; Núncio, M.S. Diagnóstico Imunológico de Doenças Associadas a Vectores Existentes Em Portugal. Boletim Epidemiológico Observações 2013, 5, 2. [Google Scholar]
- Cordero, A.; Escoto, V.; Lopes, L. Bartonella Endocarditis: A Culture-Negative Endocarditis Clinical Case Report. Port. Online J. Med. Cases Rep. 2008, 15, 3. [Google Scholar]
- Murinello, N.; Murinello, A.; Damasio, H.; Carvalho, A.; de Sousa, R. Doença Da Arranhadela Do Gato Em Mulher de 44 Anos de Idade. Rev. Port. Doenças Infecc. 2010, 6, 112–119. [Google Scholar]
- Dias, A.; Pinto, D.; Borges, T.; Guedes, M. Manifestação Atípica de Infecção Por Bartonella henselae. Port. J. Pediatrics 2014, 42, 277–279. [Google Scholar] [CrossRef]
- Mazurek, Ł.; Winiarczyk, S.; Adaszek, Ł. Feline Bartonellosis Key Issues and Possible Vectors. Ann. Parasitol. 2018, 64, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Karasartova, D.; Gureser, A.S.; Gokce, T.; Celebi, B.; Yapar, D.; Keskin, A.; Celik, S.; Ece, Y.; Erenler, A.K.; Usluca, S.; et al. Bacterial and Protozoal Pathogens Found in Ticks Collected from Humans in Corum Province of Turkey. PLoS Negl. Trop. Dis. 2018, 12, e0006395. [Google Scholar] [CrossRef] [PubMed]
- Rogovskyy, A.; Batool, M.; Gillis, D.C.; Holman, P.J.; Nebogatkin, I.V.; Rogovska, Y.V.; Rogovskyy, M.S. Diversity of Borrelia Spirochetes and Other Zoonotic Agents in Ticks from Kyiv, Ukraine. Ticks Tick Borne Dis. 2018, 9, 404–409. [Google Scholar] [CrossRef]
- Sacristán, C.; das Neves, C.G.; Suhel, F.; Sacristán, I.; Tengs, T.; Hamnes, I.S.; Madslien, K. Bartonella spp. Detection in Ticks, Culicoides Biting Midges and Wild Cervids from Norway. Transbound Emerg. Dis. 2021, 68, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Liodaki, M.; Spanakos, G.; Samarkos, M.; Daikos, G.L.; Christopoulou, V.; Piperaki, E.-T. Molecular Screening of Cat and Dog Ectoparasites for the Presence of Bartonella spp. in Attica, Greece. Acta Vet. Hung. 2022, 70, 9–14. [Google Scholar] [CrossRef]
- Telford, S.R.; Wormser, G.P. Bartonella spp. Transmission by Ticks Not Established. Emerg. Infect. Dis. 2010, 16, 379–384. [Google Scholar] [CrossRef]
- Król, N.; Militzer, N.; Stöbe, E.; Nijhof, A.M.; Pfeffer, M.; Kempf, V.A.J.; Obiegala, A. Evaluating Transmission Paths for Three Different Bartonella spp. in Ixodes ricinus Ticks Using Artificial Feeding. Microorganisms 2021, 9, 901. [Google Scholar] [CrossRef]
- Reis, C.; Cote, M.; Le Rhun, D.; Lecuelle, B.; Levin, M.L.; Vayssier-Taussat, M.; Bonnet, S.I. Vector Competence of the Tick Ixodes ricinus for Transmission of Bartonella birtlesii. PLoS Negl. Trop. Dis. 2011, 5, e1186. [Google Scholar] [CrossRef] [Green Version]
- Wechtaisong, W.; Bonnet, S.I.; Chomel, B.B.; Lien, Y.-Y.; Chuang, S.-T.; Tsai, Y.-L. Investigation of Transovarial Transmission of Bartonella henselae in Rhipicephalus sanguineus Sensu Lato Ticks Using Artificial Feeding. Microorganisms 2021, 9, 2501. [Google Scholar] [CrossRef]
- Wechtaisong, W.; Bonnet, S.I.; Lien, Y.Y.; Chuang, S.T.; Tsai, Y.L. Transmission of Bartonella henselae within Rhipicephalus sanguineus: Data on the Potential Vector Role of the Tick. PLoS Negl. Trop. Dis. 2020, 14, e0008664. [Google Scholar] [CrossRef]
- Aeschlimann, A. Ixodes ricinus, Linné, 1758 (Ixodoidea; Ixodidae). Essai Préliminaire de Synthèse Sur La Biologie de Cette Espèce En Suisse. Acta Trop. 1972, 29, 321–340. [Google Scholar]
- Santos-Silva, M.M.; Beati, L.; Santos, A.S.; De Sousa, R.; Núncio, M.S.; Melo, P.; Santos-Reis, M.; Fonseca, C.; Formosinho, P.; Vilela, C.; et al. The Hard-Tick Fauna of Mainland Portugal (Acari: Ixodidae): An Update on Geographical Distribution and Known Associations with Hosts and Pathogens. Exp. Appl. Acarol. 2011, 55, 85–121. [Google Scholar] [CrossRef]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari, Ixodidae); Cambridge University Press: Cambridge, UK, 2000; ISBN 9780521480086. [Google Scholar]
- Kosoy, M.Y.; Regnery, R.L.; Tzianabos, T.; Marston, E.L.; Jones, D.C.; Green, D.; Maupin, G.O.; Olson, J.G.; Childs, J.E. Distribution, Diversity, and Host Specificity of Bartonella in Rodents from the Southeastern United States. Am. J. Trop. Med. Hyg. 1997, 57, 578–588. [Google Scholar] [CrossRef]
- Riess, T.; Dietrich, F.; Schmidt, K.V.; Kaiser, P.O.; Schwarz, H.; Schäfer, A.; Kempf, V.A.J. Analysis of a Novel Insect Cell Culture Medium-Based Growth Medium for Bartonella Species. Appl. Environ. Microbiol. 2008, 74, 5224–5227. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, R.; Vayssier-Taussat, M.; Buffet, J.P.; Harrus, S. Guidelines for the Isolation, Molecular Detection, and Characterization of Bartonella Species. Vector-Borne Zoonotic Dis. 2017, 17, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Alekseev, A.N.; Dubinina, H.V.; Van De Pol, I.; Schouls, L.M. Identification of Ehrlichia spp. and Borrelia burgdorferi in Ixodes Ticks in the Baltic Regions of Russia. J. Clin. Microbiol. 2001, 39, 2237–2242. [Google Scholar] [CrossRef] [Green Version]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and Evaluation of a Seminested PCR for Detection and Differentiation of Babesia gibsoni (Asian Genotype) and B. canis DNA in Canine Blood Samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef] [Green Version]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. 18S RRNA Gene Sequences and Phylogenetic Relationships of European Hard-Tick Species (Acari: Ixodidae). Parasitol. Res. 1998, 84, 31–37. [Google Scholar] [CrossRef]
- André, M.R.; Dumler, J.S.; Herrera, H.M.; Gonçalves, L.R.; de Sousa, K.C.M.; Scorpio, D.G.; de Santis, A.C.G.A.; Domingos, I.H.; de Macedo, G.C.; Machado, R.Z. Assessment of a Quantitative 5’ Nuclease Real-Time Polymerase Chain Reaction Using the Nicotinamide Adenine Dinucleotide Dehydrogenase Gamma Subunit (NuoG) for Bartonella Species in Domiciled and Stray Cats in Brazil. J. Feline Med. Surg. 2016, 18, 783–790. [Google Scholar] [CrossRef]
- Billeter, S.A.; Cáceres, A.G.; Gonzales-Hidalgo, J.; Luna-Caypo, D.; Kosoy, M.Y. Molecular Detection of Bartonella Species in Ticks from Peru. J. Med. Entomol. 2011, 48, 1257–1260. [Google Scholar] [CrossRef]
- Johnson, G.; Ayers, M.; McClure, S.C.C.; Richardson, S.E.; Tellier, R. Detection and Identification of Bartonella Species Pathogenic for Humans by PCR Amplification Targeting the Riboflavin Synthase Gene (RibC). J. Clin. Microbiol. 2003, 41, 1069–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Rozas, J.; Rozas, R. DnaSP, DNA Sequence Polymorphism: An Interactive Program for Estimating Population Genetics Parameters from DNA Sequence Data. Comput. Appl. Biosci. 1995, 11, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H.A.; Strimmer, K.; Vingron, M.; Von Haeseler, A. TREE-PUZZLE: Maximum Likelihood Phylogenetic Analysis Using Quartets and Parallel Computing. Bioinformatics 2002, 18, 502–504. [Google Scholar] [CrossRef] [Green Version]



| Number of Samples | Female | Male | Adult | Juvenile | Flea Infested | |
|---|---|---|---|---|---|---|
| Cats | 123 | 73 (58.8%) | 51 (45.16%) | 112 (91.05%) | 11 (8.87%) | 9 (7.25%) |
| Dogs | 25 | 10 (43.47%) | 15 (56.52%) | 11 (47.82%) | 12 (52.17%) | 4 (17.39%) |
| Type of Samples | Efficiency (Average) | R2 (Average) | Slope (Average) | Quantification |
|---|---|---|---|---|
| Ticks | 101.98% | 0.996 | −3.285 | - |
| Cats | 94.78% | 0.994 | −3.449 | 2.78 × 10 to 1.03 × 105 copies/µL |
| Dogs | 95.25% | 0.996 | −3.448 | - |
| Humans | 106.3% | 0.678 | −3.187 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrejón, E.; Sanches, G.S.; Moerbeck, L.; Santos, L.; André, M.R.; Domingos, A.; Antunes, S. Molecular Survey of Bartonella Species in Stray Cats and Dogs, Humans, and Questing Ticks from Portugal. Pathogens 2022, 11, 749. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens11070749
Torrejón E, Sanches GS, Moerbeck L, Santos L, André MR, Domingos A, Antunes S. Molecular Survey of Bartonella Species in Stray Cats and Dogs, Humans, and Questing Ticks from Portugal. Pathogens. 2022; 11(7):749. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens11070749
Chicago/Turabian StyleTorrejón, Estefania, Gustavo Seron Sanches, Leonardo Moerbeck, Lenira Santos, Marcos Rogério André, Ana Domingos, and Sandra Antunes. 2022. "Molecular Survey of Bartonella Species in Stray Cats and Dogs, Humans, and Questing Ticks from Portugal" Pathogens 11, no. 7: 749. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens11070749