Effects of Shape and Size of Cobalt Phosphate Nanoparticles against Acanthamoeba castellanii
Abstract
:1. Introduction
2. Results
2.1. X-ray Diffraction (XRD)
2.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray (EDX)
2.3. Cobalt nanoparticles Exhibited Amoebicidal Effects Against A. castellanii
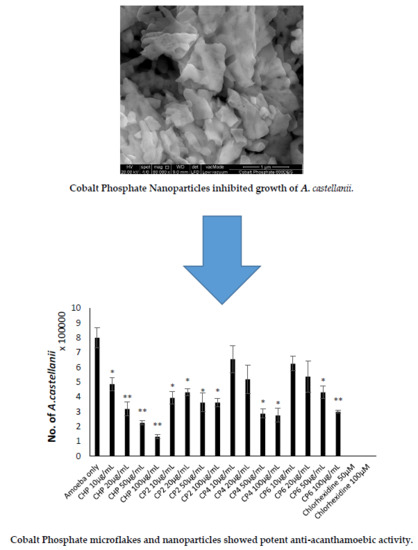
2.4. Cobalt nanoparticles Inhibited Growth of A. castellanii
2.5. Cobalt nanoparticles Suppressed Encystation Effects of A. castellanii
2.6. Cobalt nanoparticles Inhibited Emergence of Trophozoites from Cysts
2.7. Cobalt nanoparticles Exhibited Low to Moderate Cytotoxic Effects against HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray (EDX)
4.3. X-ray Diffraction (XRD)
4.4. Henrietta Lacks Cervical Adenocarcinoma (HeLa) Cells Culture
4.5. Acanthamoeba Cultures
4.6. Preparation of Cysts
4.7. Amoebicidal Assay
4.8. Growth Inhibition Assay
4.9. Encystation Assay
4.10. Excystation Assay
4.11. Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, N.A. Pathogenesis of Acanthamoeba infections. Microb. Pathog. 2003, 34, 277–285. [Google Scholar] [CrossRef]
- Khan, N.A. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 546–595. [Google Scholar] [CrossRef] [PubMed]
- Byers, T.; Kim, B.; King, L.; Hugo, E. Molecular aspects of the cell cycle and encystment of Acanthamoeba. Rev. Infect. Dis. 1991, 13, S373–S384. [Google Scholar] [CrossRef] [PubMed]
- Mazur, T.; Hadas, E.; Iwanicka, I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop. Med. Parasitol. 1995, 46, 106–108. [Google Scholar] [PubMed]
- Gast, R.J.; Ledee, D.R.; Fuerst, P.A.; Byers, T. Subgenus systematics of Acanthamoeba: Four nuclear 18S rDNA sequence types. J. Eukaryot. Microbiol. 1996, 43, 498–504. [Google Scholar] [CrossRef]
- Fuerst, P.; Booton, G.; Crary, M. Phylogenetic analysis and the evolution of the 18S rRNA gene typing system of Acanthamoeba. J. Eukaryot. Microbiol. 2015, 62, 69–84. [Google Scholar] [CrossRef]
- Visvesvara, G.; Moura, H.; Schuster, F. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [Google Scholar] [CrossRef]
- Siddiqui, R.; Khan, N. Biology and pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef]
- Martinez, A.; Janitschke, K. Acanthamoeba, an opportunistic microorganism: A review. Infection 1985, 13, 251–256. [Google Scholar] [CrossRef]
- Helton, J.; Loveless, M.; White, C.J. Cutaneous Acanthamoeba infection associated with leukocytoclastic vasculitis in an AIDS patient. Am. J. Dermatopahtol. 1993, 15, 146–149. [Google Scholar] [CrossRef]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as Agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.; Visvesvara, G. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int. J. Parasitol. 2004, 34, 1001–1027. [Google Scholar] [CrossRef] [PubMed]
- Stothard, D.R.; Hay, J.; Schroeder-Diedrich, J.M.; Seal, D.V.; Byers, T.J. Fluorescent oligonucleotide probes for clinical and environmental detection of Acanthamoeba and the T4 18S rRNA gene sequence type. J. Clin. Microbiol. 1999, 37, 2687–2693. [Google Scholar] [PubMed]
- Maudgal, P. Acanthamoeba keratitis: Report of three cases. Bull. Soc. Belge. Ophtalml. 1989, 231, 135–148. [Google Scholar]
- Tay-Kearney, M.L.; McGhee, C.N.; Crawford, G.J.; Trown, K. Acanthamoeba keratitis: A masquerade of presentation in six cases. Aust. N. Z. J. Ophtalmol. 1993, 21, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.A.; Kashiwabuchi, R.T.; Martins, S.A.; Castro-Combs, J.M.; Kalyani, S.; Stanley, P.; Flikier, D.; Behrens, A. Riboflavin and ultraviolet light a therapy as an adjuvant treatment for medically refractive Acanthamoeba keratitis: Report of 3 cases. Ophthalmology 2011, 118, 324–331. [Google Scholar] [CrossRef]
- Seal, D.; Hay, J.; Kirkess, C. Chlorhexidine or polyhexamethylene biguanide for Acanthamoeba keratitis. Lancet 1995, 345, 136. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsumoto, Y.; Kabata, T.; Watanabe, R.; Hommura, S.; Yasuraoka, K.; Ishii, K. Oral itraconazole and topical miconazole with debridement for Acanthamoeba keratitis. Am. J. Ophtalmol. 1990, 109, 121–126. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioalied. Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Rostami-Hodjegan, A.; Tucker, G. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat. Rev. Drug Discov. 2007, 6, 140–148. [Google Scholar] [CrossRef]
- Subramani, K.; Ahmed, W.; Hartsfield, J., Jr. Nanobiomaterials in Clinical Densistry; William Andrew: Norwich, UK, 2013. [Google Scholar]
- Aqeel, Y.; Siddiqui, R.; Anwar, A.; Shah, M.R.; Khan, N.A. Gold nanoparticle conjugation enhances the antiacanthamoeba effects of chlorhexidine. Anitmicrob. Agent Chemother. 2016, 60, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold nanoparticle-conjugated cinnamic acid exhibits anti-acanthamoebic and antibacterial properties. Antimicrob. Agent Chemother. 2018, 62, 1–7. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Hussain, M.A.; Ahmed, D.; Shah, M.R.; Khan, N.A. Silver nanoparticle conjugation affects anti-acanthamoebic activities of amphotericin B, nystatin, and fluconazole. Parasitol. Res. 2018, 117, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Muazzam, A.G.; Habib, A.; Matin, A. Synthesis, characterization and amoebicidal potential of locally synthesized TiO2 nanoparticles against pathogenic Acanthamoeba trophozoites in vitro. J. Photochem. Photobiol. B 2016, 159, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Niyyati, M.; Sasani, R.; Mohebali, M.; Ghazikhansari, M.; Kargar, F.; Hajialilo, E.; Rezaeian, M. Anti-Acanthamoeba effects of silver and gold nanoparticles and contact lenses disinfection solutions. Iran. J. Parasitol. 2018, 13, 180. [Google Scholar]
- O’Leary, F.; Samman, S. Vitamin B12 in Health and Disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef]
- Alahmadi, N.S.; Betts, J.W.; Cheng, F.; Francesconi, M.G.; Kelly, S.M.; Kornherr, A.; Prior, T.J.; Wadhawan, J.D. Synthesis and antibacterial effects of cobalt–cellulose magnetic nanocomposites. RSC Adv. 2017, 7, 20020–20026. [Google Scholar] [CrossRef]
- Raza, M.; Kanwal, Z.; Riaz, S.; Naseem, S. Synthesis, Characterization and Antibacterial Properties of Nano-Sized Cobalt Particles. In Proceedings of the 2016 World Congress on Advances in Civil, Enviromental, and Materials Research (ACEM16), Jeju Island, Korea, 28 August–1 September 2016; Available online: https://pdfs.semanticscholar.org/6b2a/51209fa14ad2628b3312b1c1de971b0b4736.pdf (accessed on 6 October 2019).
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K.F. Antifungal Activity and Mechanism of Action of the Co(III) Coordination Complexes With Diamine Chelate Ligands Against Reference and Clinical Strains of Candida spp. Front. Micriobiol. 2018, 9, 1594. [Google Scholar] [CrossRef]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt Complexes as Aniviral and Antibacterial Agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef]
- Marimuthu, S.; Rahuman, A.A.; Kirthi, A.V.; Santhoshkumar, T.; Jayaseelan, C.; Rajakumar, G. Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitol. Res. 2013, 112, 4105–4112. [Google Scholar] [CrossRef]
- Martín-Navarro, C.M.; López-Arencibia, A.; Lorenzo-Morales, J.; Oramas-Royo, S.; Hernández-Molina, R.; Estévez-Braun, A.; Ravelo, Á.G.; Valladares, B.; Piñero, J.E. Acanthamoeba castellanii Neff: In vitro activity against the trophozoite stage of a natural sesquiterpene and a synthetic cobalt(II)–lapachol complex. Exp. Parasitol. 2010, 126, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Numan, A.; Siddiqui, R.; Khalid, M.; Khan, N.A. Cobalt nanoparticles as novel nanotherapeutics against Acanthamoeba castellanii. Parasit. Vectors 2019, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.K.; Shahi, A.K.; Srivastava, N.; Kumar, G.; Gopal, R. Synthesis and cytogenic effect of magnetic nanoparticles. Adv. Mater. Lett. 2015, 6, 954–960. [Google Scholar] [CrossRef]
- Ahmed, D.; Anwar, A.; Khan, A.K.; Ahmed, A.; Shah, M.R.; Khan, N.A. Size selectivity in antibiofilm activity of 3-(diphenylphosphino) propanoic acid coated gold nanomaterials against Gram-positive Staphylococcus aureus and Streptococcus mutans. AMB Express 2017, 7, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedziora, A.; Strek, W.; Kepinski, L.; Bugla-Ploskonska, G.; Doroszkiewicz, W. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J. Sol-Gel Sci. Technol. 2012, 62, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Prema, P. In-vitro antibacterial activity of gold nanoparticles capped with polysaccharide stabilising agents. Int. J. Pharm. Pharm. Sci. 2013, 5, 310–314. [Google Scholar]
- Chohan, Z.; Praveen, M. Synthesis, characterization and antibacterial properties of symmetric 1,1′-ferrocene derived Schiff-base ligands and their Co(II), Cu(II), Ni(II) and Zn(II) chelates. Appl. Organometal. Chem. 2000, 14, 85–90. [Google Scholar] [CrossRef]
- Sissons, J.; Alsam, S.; Stins, M.; Rivas, A.O.; Morales, J.L.; Faull, J.; Khan, N.A. Use of in vitro assays to determine effects to human serum on biological characteristics of Acanthamoeba castellanii. J. Clin. Microbiol. 2006, 44, 2595–2600. [Google Scholar] [CrossRef] [Green Version]
- Baig, A.M.; Iqbal, J.; Khan, N.A. In vitro Efficacies of Clinically Available Drugs against Growth and Viability of an Acanthamoeba castellanii Keratitis Isolate Belonging to the T4 Genotype. Antimicrob. Agents Chemother. 2013, 57, 3561–3567. [Google Scholar] [CrossRef] [Green Version]
- Dudley, R.; Jarroll, E.L.; Khan, N.A. Carbohydrate analysis of Acanthamobe castellanii. Exp. Parasitol. 2009, 122, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Rajendran, K.; Siddiqui, R.; Raza Shah, M.; Khan, N.A. Clinically approved drugs against CNS diseases as potential therapeutic agents to target brain-eating amoebae. ACS Chem. Neurosci. 2018, 10, 658–666. [Google Scholar] [CrossRef] [PubMed]








| Elemental Atomic Percentage (at %) | CHP | CP2 | CP4 | CP6 |
|---|---|---|---|---|
| Oxygen | 78.26 | 73.05 | 69.76 | 68.99 |
| Phosphorus | 9.74 | 12.87 | 14.03 | 14.84 |
| Cobalt | 12.00 | 14.08 | 16.21 | 16.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, A.; Chi Fung, L.; Anwar, A.; Jagadish, P.; Numan, A.; Khalid, M.; Shahabuddin, S.; Siddiqui, R.; Khan, N.A. Effects of Shape and Size of Cobalt Phosphate Nanoparticles against Acanthamoeba castellanii. Pathogens 2019, 8, 260. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens8040260
Anwar A, Chi Fung L, Anwar A, Jagadish P, Numan A, Khalid M, Shahabuddin S, Siddiqui R, Khan NA. Effects of Shape and Size of Cobalt Phosphate Nanoparticles against Acanthamoeba castellanii. Pathogens. 2019; 8(4):260. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens8040260
Chicago/Turabian StyleAnwar, Ayaz, Leong Chi Fung, Areeba Anwar, Priyanka Jagadish, Arshid Numan, Mohammad Khalid, Syed Shahabuddin, Ruqaiyyah Siddiqui, and Naveed Ahmed Khan. 2019. "Effects of Shape and Size of Cobalt Phosphate Nanoparticles against Acanthamoeba castellanii" Pathogens 8, no. 4: 260. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens8040260