Distribution of Parasitic Helminths in the Small Intestine of the Red Fox (Vulpes vulpes)
Abstract
:1. Introduction
2. Results
2.1. Prevalence of Intestinal Helminths in Lubelskie Province
2.2. Analysis of Location and Distribution of Individual Parasites in the Small Intestine
- Echinococcus multilocularis
- Alaria alata
- Mesocestoides spp.
- Taenia spp.
- Hookworms (Uncinaria/Ancylostoma)
- Toxocara/Toxascaris
2.3. Comparisons of Distributions between Parasite Species
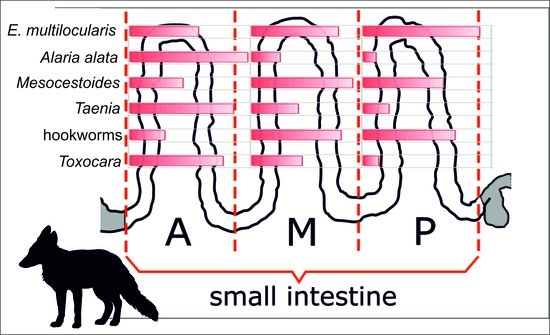
2.3.1. Occurrence in Individual Parts—Comparison between Parasites (Figure 2)
2.3.2. Comparison of Distribution Variants between Individual Parasite Species (Table 5)
2.3.3. Relationships between the Intensity of Different Parasites Existing in the Same Intestines
3. Discussion
3.1. Prevalence
3.2. Location and Distribution in the Small Intestine
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Franssen, F.; Nijsse, R.; Mulder, J.; Cremers, H.; Dam, C.; Takumi, K.; van der Giessen, J. Increase in number of helminth species from Dutch red foxes over a 35-year period. Parasites Vectors 2014, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magi, M.; Guardone, L.; Mignone, W.; Prati, M.C.; Macchioni, F. Intestinal helminths of red foxes (Vulpes vulpes) in north-west Italy. Helminthologia 2016, 53, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Bruzinskaite-Schmidhalter, R.; Sarkunas, M.; Malakauskas, A.; Mathis, A.; Torgerson, P.R.; Deplazes, P. Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Parasitology 2012, 139, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, A.; Siles-Lucas, M.; Karamon, J.; Possenti, A.; Conraths, F.J.; Romig, T.; Wysocki, P.; Mannocci, A.; Mipatrini, D.; La Torre, G.; et al. The geographical distribution and prevalence of Echinococcus multilocularis in animals in the European Union and adjacent countries: A systematic review and meta-analysis. Parasites Vectors 2016, 9, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romig, T. Echinococcus multilocularis in Europe—state of the art. Vet. Res. Commun. 2009, 33, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Wahlstrom, H.; Isomursu, M.; Hallgren, G.; Christensson, D.; Cedersmyg, M.; Wallensten, A.; Hjertqvist, M.; Davidson, R.K.; Uhlhorn, H.; Hopp, P. Combining information from surveys of several species to estimate the probability of freedom from Echinococcus multilocularis in Sweden, Finland and mainland Norway. Acta Vet. Scand. 2011, 53. [Google Scholar] [CrossRef] [Green Version]
- Ammann, R.W.; Eckert, J. Cestodes—Echinococcus. Gastroenterol. Clin. N. Am. 1996, 25, 655. [Google Scholar] [CrossRef]
- Fiocchi, A.; Gustinelli, A.; Gelmini, L.; Rugna, G.; Renzi, M.; Fontana, M.C.; Poglayen, G. Helminth parasites of the red fox Vulpes vulpes (L., 1758) and the wolf Canis lupus italicus Altobello, 1921 in Emilia-Romagna, Italy. Ital. J. Zoolog. 2016, 83, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Al-Sabi, M.N.S.; Chriél, M.; Jensen, T.H.; Enemark, H.L. Endoparasites of the raccoon dog (Nyctereutes procyonoides) and the red fox (Vulpes vulpes) in Denmark 2009–2012—A comparative study. Int. J. Parasit. Parasites Wildl. 2013, 2, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Shimalov, V.V.; Shimalov, V.T. Helminth fauna of the red fox (Vulpes vulpes Linnaeus, 1758) in southern Belarus. Parasitol. Res. 2003, 89, 77–78. [Google Scholar] [CrossRef]
- Szell, Z.; Tolnai, Z.; Sreter, T. Environmental determinants of the spatial distribution of Mesocestoides spp. and sensitivity of flotation method for the diagnosis of mesocestoidosis. Vet. Parasitol. 2015, 212, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.K.; Shimalov, V.V. A comparative study of helminths of raccoon dogs (Nyctereutes procynoides) and red foxes (Vulpes vulpes) sharing the same territory. Asian Pac. J. Trop. Dis. 2017, 7, 708–714. [Google Scholar] [CrossRef]
- Saeed, I.; Maddox-Hyttel, C.; Monrad, J.; Kapel, C.M.O. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet. Parasitol. 2006, 139, 168–179. [Google Scholar] [CrossRef]
- Karamon, J.; Dabrowska, J.; Kochanowski, M.; Samorek-Pierog, M.; Sroka, J.; Rozycki, M.; Bilska-Zajac, E.; Zdybel, J.; Cencek, T. Prevalence of intestinal helminths of red foxes (Vulpes vulpes) in central Europe (Poland): A significant zoonotic threat. Parasites Vectors 2018, 11, 36. [Google Scholar] [CrossRef]
- Saeed, I.S.; Kapel, C.M.O. Population dynamics and epidemiology of Toxocara canis in Danish red foxes. J. Parasitol. 2006, 92, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Vergles Rataj, A.; Posedi, J.; Zele, D.; Vengust, G. Intestinal parasites of the red fox (Vulpes vulpes) in Slovenia. Acta. Vet. Hung. 2013, 61, 454–462. [Google Scholar] [CrossRef]
- Smith, G.C.; Gangadharan, B.; Taylor, Z.; Laurenson, M.K.; Bradshaw, H.; Hide, G.; Hughes, J.M.; Dinkel, A.; Romig, T.; Craig, P.S. Prevalence of zoonotic important parasites in the red fox (Vulpes vulpes) in Great Britain. Vet. Parasitol. 2003, 118, 133–142. [Google Scholar] [CrossRef]
- Manke, K.J.; Stoye, M. Parasitological studies of red foxes (Vulpes vulpes L.) in the northern districts of Schleswig-Holstein. Tierarztl. Umschau. 1998, 53, 207–214. [Google Scholar]
- Al-Sabi, M.N.S.; Halasa, T.; Kapel, C.M.O. Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitol. 2014, 59, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Demkowska-Kutrzepa, M.; Szczepaniak, K.; Dudko, P.; Roczen-Karczmarz, M.; Studzinska, M.; Zyla, S.; Tomczuk, K. Determining the occurrence of the Uncinaria stenocephala and Ancylostoma caninum nematode invasion in dogs in Poland, with special emphasis on the Lublin region. Med. Weter. 2018, 74, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Tylkowska, A.; Pilarczyk, B.; Pilarczyk, R.; Zysko, M.; Tomza-Marciniak, A. The presence of Alaria alata fluke in the red fox (Vulpes vulpes) in north-western Poland. Jpn. J. Vet. Res. 2018, 66, 203–208. [Google Scholar] [CrossRef]
- Szell, Z.; Tolnai, Z.; Sreter, T. Environmental determinants of the spatial distribution of Alaria alata in Hungary. Vet. Parasitol. 2013, 198, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Karamon, J.; Samorek-Pierog, M.; Moskwa, B.; Rozycki, M.; Bilska-Zajac, E.; Zdybel, J.; Wlodarczyk, M. Intestinal helminths of raccoon dogs (Nyctereutes procyonoides) and red foxes (Vulpes vulpes) from the Augustow Primeval Forest (north-eastern Poland). J. Vet. Res. 2016, 60, 273–277. [Google Scholar] [CrossRef] [Green Version]
- Behnke, J.M.; Gilbert, F.S.; Abu-Madi, M.A.; Lewis, J.W. Do the helminth parasites of wood mice interact? J. Anim. Ecol. 2005, 74, 982–993. [Google Scholar] [CrossRef]
- Behnke, J.M.; Bajer, A.; Sinski, E.; Wakelin, D. Interactions involving intestinal nematodes of rodents experimental and field studies. Parasitology 2001, 122, S39–S49. [Google Scholar] [CrossRef] [Green Version]
- Murphy, L.; Pathak, A.K.; Cattadori, I.M. A co-infection with two gastrointestinal nematodes alters host immune responses and only partially parasite dynamics. Parasite Immunol. 2013, 35, 421–432. [Google Scholar] [CrossRef]
- Reynolds, L.A.; Finlay, B.B.; Maizels, R.M. Cohabitation in the Intestine: Interactions among Helminth Parasites, Bacterial Microbiota, and Host Immunity. J. Immunol. 2015, 195, 4059–4066. [Google Scholar] [CrossRef] [Green Version]
- Tackmann, K.; Mattis, R.; Conraths, F.J. Detection of Echinococcus multilocularis in foxes: Evaluation of a protocol of the intestinal scraping technique. J. Vet. Med. Ser. B-Infect. Dis. Vet. 2006, 53, 395–398. [Google Scholar] [CrossRef]
- Umhang, G.; Woronoff-Rhen, N.; Combes, B.; Boue, F. Segmental Sedimentation and Counting Technique (SSCT): An adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp. Parasitol. 2011, 128, 57–60. [Google Scholar] [CrossRef]
- Tylkowska, A.; Pilarczyk, B.; Pilarczyk, R.; Zysko, M.; Tomza-Marciniak, A. Presence of tapeworms (Cestoda) in red fox (Vulpes vulpes) in north-western Poland, with particular emphasis on Echinococcus multilocularis. J. Vet. Res. 2019, 63, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Borecka, A.; Gawor, J.; Malczewska, M.; Malczewski, A. Prevalence of zoonotic helminth parasites of the small intestine in red foxes from central Poland. Med. Weter. 2009, 65, 33–35. [Google Scholar]
- Karamon, J.; Sroka, J.; Cencek, T.; Michalski, M.M.; Zieba, P.; Karwacki, J. Prevalence of Echinococcus multilocularis in red foxes in two eastern provinces of Poland. B. Vet. I. Pulawy 2011, 55, 429–433. [Google Scholar]
- Borecka, A.; Gawor, J.; Malczewska, M.; Malczewski, A. Prevalence of Echinococcus multilocularis tapeworm in red foxes in central Poland. Med. Weter. 2007, 63, 1333–1335. [Google Scholar]
- Ozolina, Z.; Bagrade, G.; Deksne, G. The host age related occurrence of Alaria alata in wild canids in Latvia. Parasitol. Res. 2018, 117, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.M.; O’Connell, J.; Berzano, M.; Dold, C.; Keegan, J.D.; McCann, A.; Murphy, D.; Holden, N.M. The prevalence and distribution of Alaria alata, a potential zoonotic parasite, in foxes in Ireland. Parasitol. Res. 2012, 111, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Jankovska, I.; Brozova, A.; Mateju, Z.; Langrova, I.; Lukesova, D.; Sloup, V. Parasites with possible zoonotic potential in the small intestines of red foxes (Vulpes vulpes) from Northwest Bohemia (CzR). Helminthologia 2016, 53, 290–293. [Google Scholar] [CrossRef] [Green Version]
- Lassnig, H.; Prosl, H.; Hinterdorfer, F. Parasites of the red fox (Vulpes vulpes) in Styria. Wien. Tierarz. Monats. 1998, 85, 116–122. [Google Scholar]
- Pacon, J.; Soltysiak, Z.; Nicpon, J.; Janczak, M. Prevalence of internal helminths in red foxes (Vulpes vulpes) in selected regions of Lower Silesia. Med. Weter. 2006, 62, 67–69. [Google Scholar]
- Bajer, A.; Alsarraf, M.; Dwuznik, D.; Mierzejewska, E.J.; Kolodziej-Sobocinska, M.; Behnke-Borowczyk, J.; Banasiak, L.; Grzybek, M.; Tolkacz, K.; Kartawik, N.; et al. Rodents as intermediate hosts of cestode parasites of mammalian carnivores and birds of prey in Poland, with the first data on the life-cycle of Mesocestoides melesi. Parasites Vectors 2020, 13, 95. [Google Scholar] [CrossRef]
- Zalesny, G.; Hildebrand, J. Molecular identification of Mesocestoides spp. from intermediate hosts (rodents) in central Europe (Poland). Parasitol. Res. 2012, 110, 1055–1061. [Google Scholar] [CrossRef]
- Balicka-Ramisz, A.; Ramisz, A.; Pilarczyk, B.; Bienko, R. Fauna of gastro-intestinal parasites in red foxes in Western Poland. Med. Weter. 2003, 59, 922–925. [Google Scholar]
- Gorski, P.; Radowanska, A.; Jaros, D.; Wisniewski, M. Molecular and morphological comparison of hookworms from genus Uncinaria invading red fox (Vulpes vulpes) and dog (Canis familiaris). Wiad. Parazytol. 2006, 52, 317–320. [Google Scholar] [PubMed]
- Karamon, J.; Kochanowski, M.; Sroka, J.; Cencek, T.; Różycki, M.; Chmurzyńska, E.; Bilska–Zając, E. The prevalence of Echinococcus multilocularis in red foxes in Poland –current results (2009–2013). Parasitol Res. 2014, 113, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamon, J.; Kochanowski, M.; Dąbrowska, J.; Sroka, J.; Różycki, M.; Bilska–Zając, E.; Cencek, T. Dynamics of Echinococcus multilocularis infection in red fox populations with high and low prevalence of this parasite in Poland (2007–2014). B. Vet. I. Pulawy 2015, 59, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Combes, B.; Comte, S.; Raton, V.; Raoul, F.; Boue, F.; Umhang, G.; Favier, S.; Dunoyer, C.; Woronoff, N.; Giraudoux, P. Westward spread of Echinococcus multilocularis in foxes, France, 2005–2010. Emerg. Infect. Dis. 2012, 18, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Miterpakova, M.; Hurnikova, Z.; Antolova, D.; Dubinsky, P. Endoparasites of red fox (Vulpes vulpes) in the Slovak Republic with the emphasis on zoonotic species Echinococcus multilocularis and Trichinella spp. Helminthologia 2009, 46, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Mideo, N. Parasite adaptations to within-host competition. Trends Parasitol. 2009, 25, 261–268. [Google Scholar] [CrossRef]
- Hellard, E.; Fouchet, D.; Vavre, F.; Pontier, D. Parasite-Parasite Interactions in the Wild: How To Detect Them? Trends Parasitol. 2015, 31, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Hofer, S.; Gloor, S.; Muller, U.; Mathis, A.; Hegglin, D.; Deplazes, P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology 2000, 120, 135–142. [Google Scholar] [CrossRef] [Green Version]
- OIE. Echinococcosis/hydatidosis (infection with Echinococcus granulosus and E. multilocularis). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 6th ed.; Office International des Epizooties: Paris, France, 2008; pp. 1–15. [Google Scholar]
- Newcombe, R.G. Two-Sided Confidence Intervals for the single proportion: Comparison of seven methods. Stat. Med. 1998, 17, 857–872. [Google Scholar] [CrossRef]




| % of Positive Foxes (95%, CI) | Mean Intensity (CV%) | |
|---|---|---|
| Echinococcus multilocularisa | 18.5 (13.9–24.2) | 507.3 (92.6) |
| Alaria alata | 78.7 (72.8–83.6) | 82.2 (145.6) |
| Mesocestoides spp. | 78.2 (72.3–83.2) | 85.0 (143.3) |
| Taenia spp. | 53.2 (46.6–59.8) | 8.9 (78.8) |
| Hookworms | 72.7 (66.4–78.2) | 9.7 (70.9) |
| Toxocara/Toxascaris | 43.1 (36.6–49.7) | 4.8 (110.5) |
| Number of Positive Foxes | Percentage of Samples (Among Positive Foxes) Containing Parasites in Individual Parts of Intestines | |||
|---|---|---|---|---|
| A | M | P | ||
| Echinococcusmultilocularis | 40 | 57.5 a | 80.0 a,b | 92.5 b |
| Alaria alata | 170 | 99.4 a | 26.5 b | 10.6 c |
| Mesocestoides spp. | 169 | 45.0 a | 92.9 b | 63.9 c |
| Taenia spp. | 115 | 87.0 a | 43.5 b | 20.0 c |
| Hookworms | 157 | 29.9 a | 82.8 b | 73.2 b |
| Toxocara/Toxascaris | 93 | 78.5 a | 46.2 b | 11.8 c |
| Mean Intensity in Different Parts of Intestines (CV%) | |||
|---|---|---|---|
| A | M | P | |
| Echinococcusmultilocularis | 121.9 a (311.5) | 199.7 a (126.2) | 300.0 a (211.3) |
| Alaria alata | 80.0 a (168.4) | 8.4 b (190.7) | 3.8 b (106.0) |
| Mesocestoides spp. | 29.6 a,c (317.0) | 54.1 b (249.9) | 33.6 b,c (351.4) |
| Taenia spp. | 7.0 a (112.5) | 5.3 a (117.0) | 2.9 a (108.3) |
| Hookworms | 2.5 a (105.5) | 5.8 b (119.1) | 5.6 b (137.0) |
| Toxocara/Toxascaris | 4.4 a (148.2) | 2.6 b (108.7) | 1.4 b (49.4) |
| Mean Intensity in Different Distribution Variants (95%, CI) | |||||||
|---|---|---|---|---|---|---|---|
| A + M + P | A + M | M + P | A + P | Only A | Only M | Only P | |
| Echinococcusmultilocularis | 915.1 a (124.5) | 24.0 a,b | 72.8 a,b (151.9) | 9.0 a,b | na | 15.5 a,b (123.2) | 19.0 b (195.6) |
| Alaria alata | 362.5 a (76.3) | 99.2 a,b (119.8) | na | 70.8 a,b (161.5) | 46.0 b (161.0) | 3.0 a,b | na |
| Mesocestoides spp. | 178.6 a (201.1) | 116.8 a,b (256.9) | 45.5 a,b (146.2) | 2.0 a,b | 2.0 a,b (86.6) | 13.6 b (196.5) | 3.4 b (119.5) |
| Taenia spp. | 19.6 a (53.9) | 12.4 a,b (85.4) | 14.6 a,b (74.7) | 9.4 a,b (86.8) | 6.0 b (121.4) | 2.2 b (73.9) | 3.5 a,b (101.0) |
| Hookworms | 18.9 a (88.3) | 6.3 a,b (49.2) | 10.9 a,b (92.1) | 3.2 a,b (40.7) | 1.6 b (83.9) | 2.6 b (70.8) | 7.3 b (193.8) |
| Toxocara/Toxascaris | 14.4 a (61.4) | 7.5 a (79.7) | na | 25.5 a,b (130.3) | 3.0 a,b (111.6) | 1.4 b (64.4) | 1.8 a,b (54.7) |
| Percentage of Samples (among Positive Foxes) Containing Parasites in Individual Variants of Distribution | ||||||
|---|---|---|---|---|---|---|
| Variants of Distribution | Echinococcus multilocularis | Alaria alata | Mesocestoides spp. | Taenia spp. | Hookworms | Toxocara/ Toxascaris |
| A + M + P | 55.0 a | 8.2 b | 30.2 c | 7.8 b | 19.1 b,c | 5.4 b |
| A + M | 2.5 a | 18.2 a | 13.0 a | 25.2 a | 4.5 b | 23.7 a |
| M + P | 15.0 a,c | 0.0 b | 28.4 a | 4.3 b,c | 40.1 a | 0.0 b |
| A + P | 2.5 a | 2.4 a | 0.6 a | 6.1 a | 3.2 a | 2.2 a |
| only A | 0.0 c | 70.6 a | 1.8 c | 49.6 b | 3.2 c | 47.3 b |
| only M | 5.0 a | 0.6 b | 21.3 a | 5.2 b | 21.0 a | 17.2 a |
| only P | 20.0 a | 0.0 c | 4.7 b,c | 1.7 b,c | 8.9 a,b | 4.3 a,b,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamon, J.; Sroka, J.; Dąbrowska, J.; Bilska-Zając, E.; Skrzypek, K.; Różycki, M.; Zdybel, J.; Cencek, T. Distribution of Parasitic Helminths in the Small Intestine of the Red Fox (Vulpes vulpes). Pathogens 2020, 9, 477. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060477
Karamon J, Sroka J, Dąbrowska J, Bilska-Zając E, Skrzypek K, Różycki M, Zdybel J, Cencek T. Distribution of Parasitic Helminths in the Small Intestine of the Red Fox (Vulpes vulpes). Pathogens. 2020; 9(6):477. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060477
Chicago/Turabian StyleKaramon, Jacek, Jacek Sroka, Joanna Dąbrowska, Ewa Bilska-Zając, Katarzyna Skrzypek, Mirosław Różycki, Jolanta Zdybel, and Tomasz Cencek. 2020. "Distribution of Parasitic Helminths in the Small Intestine of the Red Fox (Vulpes vulpes)" Pathogens 9, no. 6: 477. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060477