Research into Neospora caninum—What Have We Learnt in the Last Thirty Years?
Abstract
:1. Introduction
2. Results
2.1. Topic Modelling
2.2. Bibliometric Analysis
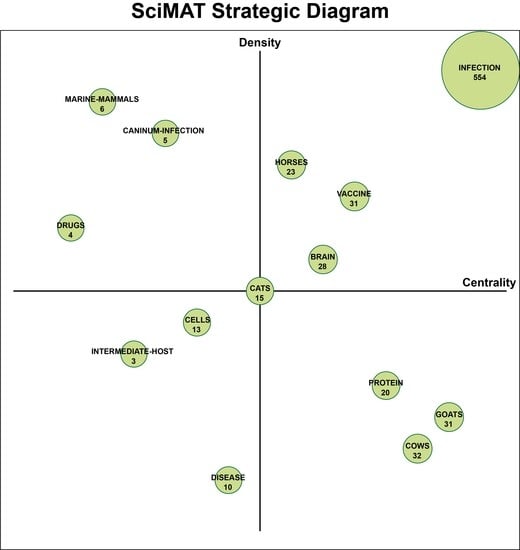
2.3. SciMAT
2.4. Systematic Review of Literature
2.4.1. Time Period 1989 to 1996 (N. caninum and Dog)
2.4.2. Time Period 1997 to 2005 (N. caninum and Abortion)
2.4.3. Time Period 2006 to 2012 (N. caninum and Seroprevalence)
2.4.4. Time Period 2013 to 2019 (N. caninum and Infection)
2.4.5. All Time Periods
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindsay, D.S.; Dubey, J.P. Immunohistochemical diagnosis of Neospora caninum in tissue sections. Am. J. Vet. Res. 1989, 50, 1981–1983. [Google Scholar]
- Thilsted, J.P.; Dubey, J.P. Neosporosis-Like Abortions in a Herd of Dairy Cattle. J. Vet. Diagn. Investig. 1989, 1, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J. Congenital neosporosis in a calf. Vet. Rec. 1989, 125, 486. [Google Scholar] [CrossRef]
- Reichel, M.P.; Ayanegui-Alcérreca, M.A.; Gondim, L.F.; Ellis, J. What is the global economic impact of Neospora caninum in cattle—The billion dollar question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, J.P.; Hemphill, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–529. [Google Scholar]
- Dubey, J.P.; Lindsay, D.S. Neospora Caninum Induced Abortion in Sheep. J. Vet. Diagn. Investig. 1990, 2, 230–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, B.C.; Anderson, M.L.; Woods, L.W.; Dubey, J.P.; Conrad, P.A. Neospora-Like Protozoal Infections Associated with Abortion in Goats. J. Vet. Diagn. Investig. 1992, 4, 365–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, S.; King, J.; Windsor, P.; Reichel, M.P.; Ellis, J.; Šlapeta, J. The first report of ovine cerebral neosporosis and evaluation of Neospora caninum prevalence in sheep in New South Wales. Vet. Parasitol. 2010, 170, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Reichel, M.P.; McAllister, M.; Nasir, A.; Moore, D. A review of Neospora caninum in water buffalo (Bubalus bubalis). Vet. Parasitol. 2015, 212, 75–79. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.; Dubey, J.P.; Lindsay, D.S.; Jolley, W.R.; Wills, R.A.; McGuire, A.M. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 1998, 28, 1473–1478. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lindsay, D.S. Transplacental Neospora caninum infection in dogs. Am. J. Vet. Res. 1989, 50, 1578–1579. [Google Scholar]
- Barber, J.S.; Trees, A.J. Clinical aspects of 27 cases of neosporosis in dogs. Vet. Rec. 1996, 139, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Gondim, L.F.; McAllister, M.; Pitt, W.C.; Zemlicka, D.E. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int. J. Parasitol. 2004, 34, 159–161. [Google Scholar] [CrossRef] [Green Version]
- King, J.S.; Šlapeta, J.; Jenkins, D.J.; Al-Qassab, S.E.; Ellis, J.; Windsor, P. Australian dingoes are definitive hosts of Neospora caninum. Int. J. Parasitol. 2010, 40, 945–950. [Google Scholar] [CrossRef]
- Dubey, J.; Buxton, D.; Wouda, W. Pathogenesis of Bovine Neosporosis. J. Comp. Pathol. 2006, 134, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.E.; Phillips, J.; Miller, K.; Tandon, A.; Mav, D.; Shah, M.R.; Holmgren, S.; Pelch, K.E.; Walker, V.; Rooney, A.A.; et al. SWIFT-Review: A text-mining workbench for systematic review. Syst. Rev. 2016, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2009, 84, 523–538. [Google Scholar] [CrossRef] [Green Version]
- Björkman, C.; Lundén, A.; Holmdahl, J.; Barber, J.; Trees, A.J.; Uggla, A. Neospora caninum in dogs: Detection of antibodies by ELISA using an iscom antigen. Parasite Immunol. 1994, 16, 643–648. [Google Scholar] [CrossRef]
- Trees, A.; Guy, F.; Tennant, B.; Balfour, A.; Dubey, J. Prevalence of antibodies to Neospora caninum in a population of urban dogs in England. Vet. Rec. 1993, 132, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Frössling, J.; Bonnett, B.; Lindberg, A.; Björkman, C. Validation of a Neospora caninum iscom ELISA without a gold standard. Prev. Vet. Med. 2003, 57, 141–153. [Google Scholar] [CrossRef]
- Björkman, C.; McAllister, M.; Frössling, J.; Näslund, K.; Leung, F.; Uggla, A. Application of the Neospora caninum IgG avidity ELISA in assessment of chronic reproductive losses after an outbreak of neosporosis in a herd of beef cattle. J. Vet. Diagn. Investig. 2003, 15, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Schares, G.; Rauser, M.; Söndgen, P.; Rehberg, P.; Bärwald, A.; Dubey, J.; Edelhofer, R.; Conraths, F.J. Use of purified tachyzoite surface antigen p38 in an ELISA to diagnose bovine neosporosis. Int. J. Parasitol. 2000, 30, 1123–1130. [Google Scholar] [CrossRef]
- Chahan, B.; Gaturaga, I.; Huang, X.; Liao, M.; Fukumoto, S.; Hirata, H.; Nishikawa, Y.; Suzuki, H.; Sugimoto, C.; Nagasawa, H.; et al. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 2003, 118, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Schares, G.; Bärwald, A.; Staubach, C.; Wurm, R.; Rauser, M.; Conraths, F.J.; Schroeder, C. Adaptation of a commercial ELISA for the detection of antibodies against Neospora caninum in bovine milk. Vet. Parasitol. 2004, 120, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Romand, S.; Thulliez, P.; Dubey, J. Direct agglutination test for serologic diagnosis of Neospora caninum infection. Parasitol. Res. 1998, 84, 50–53. [Google Scholar] [CrossRef]
- Álvarez-García, G.; Collantes-Fernandez, E.; Costas, E.; Rebordosa, X.; Ortega-Mora, L.M. Influence of age and purpose for testing on the cut-off selection of serological methods in bovine neosporosis. Vet. Res. 2003, 34, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-García, G.; Pereira-Bueno, J.; Gómez-Bautista, M.; Ortega-Mora, L.M. Pattern of recognition of Neospora caninum tachyzoite antigens by naturally infected pregnant cattle and aborted foetuses. Vet. Parasitol. 2002, 107, 15–27. [Google Scholar] [CrossRef]
- Van Maanen, C.; Wouda, W.; Schares, G.; Von Blumröder, D.; Conraths, F.J.; Norton, R.; Williams, D.; Esteban-Redondo, I.; Innes, E.A.; Mattsson, J.; et al. An interlaboratory comparison of immunohistochemistry and PCR methods for detection of Neospora caninum in bovine foetal tissues. Vet. Parasitol. 2004, 126, 351–364. [Google Scholar] [CrossRef]
- Collantes-Fernández, E.; Zaballos, Á.; Álvarez-García, G.; Ortega-Mora, L.M. Quantitative Detection of Neospora caninum in Bovine Aborted Fetuses and Experimentally Infected Mice by Real-Time PCR. J. Clin. Microbiol. 2002, 40, 1194–1198. [Google Scholar] [CrossRef] [Green Version]
- Sager, H.; Fischer, I.; Furrer, K.; Strasser, M.; Waldvogel, A.; Boerlin, P.; Audigé, L.; Gottstein, B. A Swiss case-control study to assess Neospora caninum-associated bovine abortions by PCR, histopathology and serology. Vet. Parasitol. 2001, 102, 1–15. [Google Scholar] [CrossRef]
- Morales-Salinas, E.; Trigo, F.; Ibarra, F.; Puente, E.; Santacruz, M. Neosporosis in Mexican Dairy Herds: Lesions and Immunohistochemical Detection of Neospora caninum in Fetuses. J. Comp. Pathol. 2001, 125, 58–63. [Google Scholar] [CrossRef]
- Wouda, W.; Moen, A.R.; Visser, I.J.R.; Van Knapen, F. Bovine fetal neosporosis: A comparison of epizootic and sporadic abortion cases and different age classes with regard to lesion severity and immunohistochemical identification of organisms in brain, heart, and liver. J. Vet. Diagn. Investig. 1997, 9, 180–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.; Reichel, M.P.; Ellis, J. Neospora abortions in dairy cattle: Diagnosis, mode of transmission and control. Vet. Parasitol. 2005, 128, 231–241. [Google Scholar] [CrossRef]
- Davison, H.C.; Otter, A.; Trees, A. Estimation of vertical and horizontal transmission parameters of Neospora caninum infections in dairy cattle. Int. J. Parasitol. 1999, 29, 1683–1689. [Google Scholar] [CrossRef]
- Schares, G.; Peters, M.; Wurm, R.; Bärwald, A.; Conraths, F.J. The efficiency of vertical transmission of Neospora caninum in dairy cattle analysed by serological techniques. Vet. Parasitol. 1998, 80, 87–98. [Google Scholar] [CrossRef]
- Thurmond, M.C.; Hietala, S. Effect of congenitally acquired Neospora caninum infection on risk of abortion and subsequent abortions in dairy cattle. Am. J. Vet. Res. 1997, 58, 1381–1385. [Google Scholar]
- Pare, J.; Thurmond, M.C.; Hietala, S.K. Neospora caninum Antibodies in Cows during Pregnancy as a Predictor of Congenital Infection and Abortion. J. Parasitol. 1997, 83, 82–87. [Google Scholar] [CrossRef]
- Pfeiffer, D.U.; Williamson, N.; Reichel, M.P.; Wichtel, J.; Teague, W. A longitudinal study of Neospora caninum infection on a dairy farm in New Zealand. Prev. Vet. Med. 2002, 54, 11–24. [Google Scholar] [CrossRef]
- Dijkstra, T.; Barkema, H.W.; Hesselink, J.; Wouda, W. Point source exposure of cattle to Neospora caninum consistent with periods of common housing and feeding and related to the introduction of a dog. Vet. Parasitol. 2002, 105, 89–98. [Google Scholar] [CrossRef]
- Dijkstra, T.; Barkema, H.W.; Eysker, M.; Wouda, W. Evidence of post-natal transmission of Neospora caninum in Dutch dairy herds. Int. J. Parasitol. 2001, 31, 209–215. [Google Scholar] [CrossRef]
- McAllister, M.M.; Björkman, C.; Anderson-Sprecher, R.; Rogers, D.G. Evidence of point-source exposure to Neospora caninum and protective immunity in a herd of beef cows. J. Am. Vet. Med. Assoc. 2000, 217, 881–887. [Google Scholar] [CrossRef]
- Atkinson, R.; Cook, R.; Reddacliff, L.; Rothwell, J.; Broady, K.; Harper, P.; Ellis, J.; Ellis, J. Seroprevalence ofNeospora caninuminfection following an abortion outbreak in a dairy cattle herd. Aust. Vet. J. 2000, 78, 262–266. [Google Scholar] [CrossRef]
- Thurmond, M.C.; Hietala, S.K.; Blanchard, P.C. Herd-Based Diagnosis of Neospora Caninum-Induced Endemic and Epidemic Abortion in Cows and Evidence for Congenital and Postnatal Transmission. J. Vet. Diagn. Investig. 1997, 9, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Bergeron, N.; Fecteau, G.; Paré, J.; Martineau, R.; Villeneuve, A. Vertical and horizontal transmission of Neospora caninum in dairy herds in Québec. Can. Vet. J. 2000, 41, 464–467. [Google Scholar]
- Moen, A.; Wouda, W.; Mul, M.; Graat, E.; Van Werven, T. Increased risk of abortion following neospora caninum abortion outbreaks: A retrospective and prospective cohort study in four dairy herds. Theriogenology 1998, 49, 1301–1309. [Google Scholar] [CrossRef]
- Bartels, C.; Wouda, W.; Schukken, Y. Risk factors for neospora caninum-associated abortion storms in dairy herds in The Netherlands (1995 to 1997). Theriogenology 1999, 52, 247–257. [Google Scholar] [CrossRef]
- Hobson, J.; Duffield, T.; Kelton, D.; Lissemore, K.; Hietala, S.; Leslie, K.; McEwen, B.; Peregrine, A. Risk factors associated with Neospora caninum abortion in Ontario Holstein dairy herds. Vet. Parasitol. 2005, 127, 177–188. [Google Scholar] [CrossRef]
- Lopez-Gatius, F.; Santolaria, P.; Yániz, J.L.; Garbayo, J.M.; Almeria, S. The Use of Beef Bull Semen Reduced the Risk of Abortion in Neospora-seropositive Dairy Cows. J. Vet. Med. Ser. B 2005, 52, 88–92. [Google Scholar] [CrossRef]
- Chi, J.; VanLeeuwen, J.A.; Weersink, A.; Keefe, G.P. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 2002, 55, 137–153. [Google Scholar] [CrossRef]
- Hobson, J.C.; Duffield, T.F.; Kelton, D.; Lissemore, K.; Hietala, S.K.; Leslie, K.E.; McEwen, B.; Cramer, G.; Peregrine, A.S. Neospora caninum serostatus and milk production of Holstein cattle. J. Am. Vet. Med Assoc. 2002, 221, 1160–1164. [Google Scholar] [CrossRef]
- Kim, J.T.; Park, J.Y.; Seo, H.S.; Oh, H.G.; Noh, J.W.; Kim, J.H.; Kim, D.Y.; Youn, H.J. In vitro antiprotozoal effects of artemisinin on Neospora caninum. Vet. Parasitol. 2002, 103, 53–63. [Google Scholar] [CrossRef]
- Gottstein, B.; Razmi, G.R.; Ammann, P.; Sager, H.; Müller, N. Toltrazuril treatment to control diaplacental Neospora caninum transmission in experimentally infected pregnant mice. Parasitology 2005, 130, 41–48. [Google Scholar] [CrossRef]
- Kritzner, S.; Sager, H.; Blum, J.; Krebber, R.; Greif, G.; Gottstein, B. An explorative study to assess the efficacy of Toltrazuril-sulfone (Ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 2002, 1, 4. [Google Scholar] [CrossRef]
- Miller, C.; Quinn, H.; Windsor, P.; Ellis, J.; Ellis, J. Characterisation of the first Australian isolate of Neospora caninum from cattle. Aust. Vet. J. 2002, 80, 620–625. [Google Scholar] [CrossRef]
- Basso, W.; Herrmann, D.; Conraths, F.J.; Pantchev, N.; Vrhovec, M.G.; Schares, G. First isolation of Neospora caninum from the faeces of a dog from Portugal. Vet. Parasitol. 2009, 159, 162–166. [Google Scholar] [CrossRef]
- Schock, A.; Innes, E.A.; Yamane, I.; Latham, S.M.; Wastling, J.M. Genetic and biological diversity among isolates of Neospora caninum. Parasitology 2001, 123, 13–23. [Google Scholar] [CrossRef]
- Williams, D.J.; Guy, C.S.; Smith, R.; Guy, F.; McGarry, J.W.; McKay, J.S.; Trees, A.J. First demonstration of protective immunity against foetopathy in cattle with latent Neospora caninum infection. Int. J. Parasitol. 2003, 33, 1059–1065. [Google Scholar] [CrossRef]
- Williams, D.J.L.; Guy, C.S.; McGarry, J.W.; Guy, F.; Tasker, L.; Smith, R.F.; MacEachern, K.; Cripps, P.J.; Kelly, D.F.; Trees, A. Neospora caninum-associated abortion in cattle: The time of experimentally-induced parasitaemia during gestation determines foetal survival. Parasitology 2000, 121, 347–358. [Google Scholar] [CrossRef]
- De Marez, T.; Liddell, S.; Dubey, J.P.; Jenkins, M.C.; Gasbarre, L. Oral infection of calves with Neospora caninum oocysts from dogs: Humoral and cellular immune responses. Int. J. Parasitol. 1999, 29, 1647–1657. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Xuan, X.; Nagasawa, H.; Igarashi, I.; Fujisaki, K.; Otsuka, H.; Mikami, T. Prevention of vertical transmission of Neospora caninum in BALB/c mice by recombinant vaccinia virus carrying NcSRS2 gene. Vaccine 2001, 19, 1710–1716. [Google Scholar] [CrossRef]
- Zúñiga, J.J.R.; Perez, E.; Frankena, K. Effect of a killed whole Neospora caninum tachyzoite vaccine on the crude abortion rate of Costa Rican dairy cows under field conditions. Vet. Parasitol. 2004, 123, 149–159. [Google Scholar] [CrossRef]
- Sadrebazzaz, A.; Haddadzadeh, H.; Esmailnia, K.; Habibi, G.; Vojgani, M.; Hashemifesharaki, R. Serological prevalence of Neospora caninum in healthy and aborted dairy cattle in Mashhad, Iran. Vet. Parasitol. 2004, 124, 201–204. [Google Scholar] [CrossRef]
- Corbellini, L.G.; Driemeier, D.; Cruz, C.F.E.; Gondim, L.F.; Wald, V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet. Parasitol. 2002, 103, 195–202. [Google Scholar] [CrossRef]
- Helmick, B.; Otter, A.; McGarry, J.; Buxton, D. Serological investigation of aborted sheep and pigs for infection by Neospora caninum. Res. Vet. Sci. 2002, 73, 187–189. [Google Scholar] [CrossRef]
- King, J.S.; Brown, G.K.; Jenkins, D.J.; Ellis, J.; Fleming, P.J.S.; Windsor, P.; Šlapeta, J. Oocysts and high seroprevalence of Neospora caninum in dogs living in remote Aboriginal communities and wild dogs in Australia. Vet. Parasitol. 2012, 187, 85–92. [Google Scholar] [CrossRef]
- Costa, K.; Santos, S.; Uzeda, R.; Pinheiro, A.M.; Almeida, M.; Araujo, F.; McAllister, M.; Gondim, L.F. Chickens (Gallus domesticus) are natural intermediate hosts of Neospora caninum. Int. J. Parasitol. 2008, 38, 157–159. [Google Scholar] [CrossRef]
- Molina-López, R.; Cabezón, O.; Pabón, M.; Darwich, L.; Obón, E.; Lopez-Gatius, F.; Dubey, J.; Almeria, S. High seroprevalence of Toxoplasma gondii and Neospora caninum in the Common raven (Corvus corax) in the Northeast of Spain. Res. Vet. Sci. 2012, 93, 300–302. [Google Scholar] [CrossRef]
- Eiras, C.; Arnaiz, I.; Álvarez-García, G.; Ortega-Mora, L.M.; Sanjuánl, M.; Yus, E.; Diéguez, F.J. Neospora caninum seroprevalence in dairy and beef cattle from the northwest region of Spain, Galicia. Prev. Vet. Med. 2011, 98, 128–132. [Google Scholar] [CrossRef]
- Panadero, R.; Painceira, A.; López, C.; Vázquez, L.; Paz, A.; Diaz, P.; DaCal, V.; Cienfuegos, S.; Fernández, P.D.; Lago, N.; et al. Seroprevalence of Toxoplasma gondii and Neospora caninum in wild and domestic ruminants sharing pastures in Galicia (Northwest Spain). Res. Vet. Sci. 2010, 88, 111–115. [Google Scholar] [CrossRef]
- Kamga-Waladjo, A.R.; Gbati, O.B.; Kone, P.; Lapo, R.A.; Chatagnon, G.; Bakou, S.N.; Pangui, L.J.; Diop, P.E.H.; Akakpo, J.A.; Tainturier, D. Seroprevalence of Neospora caninum antibodies and its consequences for reproductive parameters in dairy cows from Dakar–Senegal, West Africa. Trop. Anim. Health Prod. 2009, 42, 953–959. [Google Scholar] [CrossRef]
- Scott, H.M.; Sorensen, O.; Wu, J.T.; Chow, E.Y.; Manninen, K.; VanLeeuwen, J.A. Seroprevalence of Mycobacterium avium subspecies paratuberculosis, Neospora caninum, Bovine leukemia virus, and Bovine viral diarrhea virus infection among dairy cattle and herds in Alberta and agroecological risk factors associated with seropositivity. Can. Vet. J. 2006, 47, 981–991. [Google Scholar]
- VanLeeuwen, J.; Tiwari, A.; Plaizier, J.C.; Whiting, T.L. Seroprevalences of antibodies against bovine leukemia virus, bovine viral diarrhea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in beef and dairy cattle in Manitoba. Can. Vet. J. 2006, 47, 783–786. [Google Scholar]
- Corbellini, L.G.; Smith, D.R.; Pescador, C.A.; Schmitz, M.; Corrêa, A.; Steffen, D.J.; Driemeier, D. Herd-level risk factors for Neospora caninum seroprevalence in dairy farms in southern Brazil. Prev. Vet. Med. 2006, 74, 130–141. [Google Scholar] [CrossRef]
- Bartels, C.; Arnaiz-Seco, J.; Ruiz-Santa-Quitera, A.; Björkman, C.; Frössling, J.; Von Blumröder, D.; Conraths, F.J.; Schares, G.; Van Maanen, C.; Wouda, W.; et al. Supranational comparison of Neospora caninum seroprevalences in cattle in Germany, The Netherlands, Spain and Sweden. Vet. Parasitol. 2006, 137, 17–27. [Google Scholar] [CrossRef]
- Ueno, T.E.H.; Gonçalves, V.S.P.; Heinemann, M.B.; Dilli, T.L.B.; Akimoto, B.M.; De Souza, S.L.P.; Gennari, S.M.; Soares, R.M. Prevalence of Toxoplasma gondii and Neospora caninum infections in sheep from Federal District, central region of Brazil. Trop. Anim. Health Prod. 2008, 41, 547–552. [Google Scholar] [CrossRef] [Green Version]
- De Craeye, S.; Speybroeck, N.; Ajzenberg, D.; Dardé, M.L.; Collinet, F.; Tavernier, P.; Van Gucht, S.; Dorny, P.; Dierick, K. Toxoplasma gondii and Neospora caninum in wildlife: Common parasites in Belgian foxes and Cervidae? Vet. Parasitol. 2011, 178, 64–69. [Google Scholar] [CrossRef]
- Almeria, S.; Vidal, D.; Ferrer, D.; Pabón, M.; De Mera, I.G.F.; Ruiz-Fons, J.F.; Alzaga, V.; Marco, I.; Calvete, C.; Lavín, S.; et al. Seroprevalence of Neospora caninum in non-carnivorous wildlife from Spain. Vet. Parasitol. 2007, 143, 21–28. [Google Scholar] [CrossRef]
- Stieve, E.; Beckmen, K.; Kania, S.A.; Widner, A.; Patton, S. Neospora caninum and toxoplasma gondii antibody prevalence in alaska wildlife. J. Wildl. Dis. 2010, 46, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Almberg, E.S.; Mech, L.D.; Smith, D.W.; Sheldon, J.W.; Crabtree, R.L. A Serological Survey of Infectious Disease in Yellowstone National Park’s Canid Community. PLoS ONE 2009, 4, e7042. [Google Scholar] [CrossRef]
- Sedlák, K.; Bártová, E. Seroprevalences of antibodies to Neospora caninum and Toxoplasma gondii in zoo animals. Vet. Parasitol. 2006, 136, 223–231. [Google Scholar] [CrossRef]
- Häsler, B.; Stärk, K.D.C.; Sager, H.; Gottstein, B.; Reist, M. Simulating the impact of four control strategies on the population dynamics of Neospora caninum infection in Swiss dairy cattle. Prev. Vet. Med. 2006, 77, 254–283. [Google Scholar] [CrossRef]
- Langoni, H.; Fornazari, F.; Da Silva, R.C.; Monti, E.T.; Villa, F.B. Prevalence of antibodies against Toxoplasma gondii and Neospora caninum in dogs. Braz. J. Microbiol. 2014, 44, 1327–1330. [Google Scholar] [CrossRef] [Green Version]
- Silaghi, C.; Knaus, M.; Rapti, D.; Kusi, I.; Shukullari, E.; Hamel, D.; Pfister, K.; Rehbein, S. Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasites Vectors 2014, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Bruhn, F.R.P.; Daher, D.O.; Lopes, E.; Barbieri, J.M.; Da Rocha, C.M.B.M.; Guimarães, A.M. Factors associated with seroprevalence of Neospora caninum in dairy cattle in southeastern Brazil. Trop. Anim. Health Prod. 2012, 45, 1093–1098. [Google Scholar] [CrossRef]
- Unzaga, J.; Moré, G.; Bacigalupe, D.; Rambeaud, M.; Pardini, L.; Dellarupe, A.; De Felice, L.; Gos, M.; Venturini, M. Toxoplasma gondii and Neospora caninum infections in goat abortions from Argentina. Parasitol. Int. 2014, 63, 865–867. [Google Scholar] [CrossRef]
- González-Warleta, M.; Castro-Hermida, J.A.; Regidor-Cerrillo, J.; Benavides, J.; Álvarez-García, G.; Fuertes, M.; Ortega-Mora, L.M.; Mezo, M. Neospora caninum infection as a cause of reproductive failure in a sheep flock. Vet. Res. 2014, 45, 88. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.K.; Li, J.Y.; Pan, H. Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infections in small ruminants in China. Prev. Vet. Med. 2015, 118, 488–492. [Google Scholar] [CrossRef]
- Ribeiro, M.J.M.; Rosa, M.H.F.; Bruhn, F.R.P.; Garcia, A.D.M.; Rocha, C.M.B.M.; Guimarães, A.M. Seroepidemiology of Sarcocystis neurona, Toxoplasma gondii and Neospora spp. among horses in the south of the state of Minas Gerais, Brazil. Rev. Bras. Parasitol. Vet. 2016, 25, 142–150. [Google Scholar] [CrossRef]
- Lempp, C.; Jungwirth, N.; Grilo, M.L.; Reckendorf, A.; Ulrich, A.; Van Neer, A.; Bodewes, R.; Pfankuche, V.M.; Bauer, C.; Osterhaus, A.D.M.E.; et al. Pathological findings in the red fox (Vulpes vulpes), stone marten (Martes foina) and raccoon dog (Nyctereutes procyonoides), with special emphasis on infectious and zoonotic agents in Northern Germany. PLoS ONE 2017, 12, e0175469. [Google Scholar] [CrossRef]
- Arranz-Solís, D.; Benavides, J.; Regidor-Cerrillo, J.; Horcajo, P.; Castaño, P.; Ferreras, M.; Jiménez-Pelayo, L.; Collantes-Fernandez, E.; Ferre, I.; Hemphill, A.; et al. Systemic and local immune responses in sheep after Neospora caninum experimental infection at early, mid and late gestation. Vet. Res. 2016, 47, 2. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ispierto, I.; Almeria, S.; Serrano-Pérez, B.; De Sousa, N.; Beckers, J.; Lopez-Gatius, F. Plasma Concentrations of Pregnancy-Associated Glycoproteins Measured Using Anti-Bovine PAG-2 Antibodies on Day 120 of Gestation Predict Abortion in Dairy Cows Naturally Infected withNeospora caninum. Reprod. Domest. Anim. 2012, 48, 613–618. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; Arranz-Solís, D.; Benavides, J.; Gómez-Bautista, M.; Castro-Hermida, J.A.; Mezo, M.; Perez, V.; Ortega-Mora, L.M.; González-Warleta, M. Neospora caninum infection during early pregnancy in cattle: How the isolate influences infection dynamics, clinical outcome and peripheral and local immune responses. Vet. Res. 2014, 45, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villagra-Blanco, R.; Silva, L.M.R.; Muñoz-Caro, T.; Yang, Z.; Li, J.; Gärtner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine Polymorphonuclear Neutrophils Cast Neutrophil Extracellular Traps against the Abortive Parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Hermosilla, C.; Taubert, A.; He, X.; Wang, X.; Gong, P.; Li, J.; Yang, Z.; Zhang, X. Canine Neutrophil Extracellular Traps Release Induced by the Apicomplexan Parasite Neospora caninum In Vitro. Front. Immunol. 2016, 7, 436. [Google Scholar] [CrossRef] [Green Version]
- Keyloun, K.R.; Reid, M.C.; Choi, R.; Song, Y.; Fox, A.M.W.; Hillesland, H.K.; Zhang, Z.; Vidadala, R.; Merritt, E.A.; Lau, A.; et al. The gatekeeper residue and beyond: Homologous calcium-dependent protein kinases as drug development targets for veterinarian Apicomplexa parasites. Parasitology 2014, 141, 1499–1509. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Aguado, A.; Laleu, B.; Balmer, V.; Ritler, D.; Hemphill, A. In vitro screening of the open source Pathogen Box identifies novel compounds with profound activities against Neospora caninum. Int. J. Parasitol. 2017, 47, 801–809. [Google Scholar] [CrossRef]
- Williams, D.J.L.; Trees, A.J. Review Article. Protecting babies: Vaccine strategies to prevent foetopathy in Neospora caninum-infected cattle. Parasite Immunol. 2006, 28, 61–67. [Google Scholar] [CrossRef]
- Williams, D.J.L.; Guy, C.S.; Smith, R.; Ellis, J.; Björkman, C.; Reichel, M.P.; Trees, A.J. Immunization of Cattle with Live Tachyzoites of Neospora caninum Confers Protection against Fetal Death. Infect. Immun. 2006, 75, 1343–1348. [Google Scholar] [CrossRef] [Green Version]
- McAllister, M. Diagnosis and Control of Bovine Neosporosis. Vet. Clin. N. Am. Food Anim. Pract. 2016, 32, 443–463. [Google Scholar] [CrossRef]
- Reichel, M.P.; Moore, D.; Hemphill, A.; Ortega-Mora, L.M.; Dubey, J.; Ellis, J. A live vaccine against Neospora caninum abortions in cattle. Vaccine 2015, 33, 1299–1301. [Google Scholar] [CrossRef]
- Goodswen, S.J.; Kennedy, P.; Ellis, J. Vacceed: A high-throughput in silico vaccine candidate discovery pipeline for eukaryotic pathogens based on reverse vaccinology. Bioinformatics 2014, 30, 2381–2383. [Google Scholar] [CrossRef] [Green Version]
- Calarco, L.; Barratt, J.; Ellis, J. Genome Wide Identification of Mutational Hotspots in the Apicomplexan Parasite Neospora caninum and the Implications for Virulence. Genome Biol. Evol. 2018, 10, 2417–2431. [Google Scholar] [CrossRef] [PubMed]
- Calarco, L.; Ellis, J. Annotating the ‘hypothetical’ in hypothetical proteins: In-silico analysis of uncharacterised proteins for the Apicomplexan parasite, Neospora caninum. Vet. Parasitol. 2019, 265, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berna, L.; Marquez, P.; Cabrera, A.; Greif, G.; Francia, M.E.; Robello, C. Reevaluation of the Toxoplasma gondii and Neospora caninum genomes reveals misassembly, karyotype differences and chromosomal rearrangements. bioRxiv 2020. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martínez, A.; Manser, V.; Wong, H.N.; Haynes, R.K.; Hemphill, A. Repurposing of antiparasitic drugs: The hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model. Vet. Res. 2016, 47, 32. [Google Scholar] [CrossRef] [Green Version]
- Hemphill, A.; Aguado-Martínez, A.; Müller, J. Approaches for the vaccination and treatment of Neospora caninum infections in mice and ruminant models. Parasitology 2015, 143, 245–259. [Google Scholar] [CrossRef]
- Duarte, P.O.; Oshiro, L.M.; Zimmermann, N.P.; Csordas, B.G.; Dourado, D.M.; Barros, J.C.; Andreotti, R. Serological and molecular detection of Neospora caninum and Toxoplasma gondii in human umbilical cord blood and placental tissue samples. Sci. Rep. 2020, 10, 9043. [Google Scholar] [CrossRef]
- Lobato, J.; Silva, D.A.O.; Mineo, T.W.P.; Amaral, J.D.H.F.; Segundo, G.R.S.; Costa-Cruz, J.M.; Ferreira, M.S.; Borges, A.S.; Mineo, J.R. Detection of Immunoglobulin G Antibodies to Neospora caninum in Humans: High Seropositivity Rates in Patients Who Are Infected by Human Immunodeficiency Virus or Have Neurological Disorders. Clin. Vaccine Immunol. 2006, 13, 84–89. [Google Scholar] [CrossRef] [Green Version]
- McCann, C.M.; Vyse, A.J.; Salmon, R.L.; Thomas, D.; Williams, D.J.; McGarry, J.W.; Pebody, R.; Trees, A. Lack of Serologic Evidence ofNeospora caninumin Humans, England. Emerg. Infect. Dis. 2008, 14, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Lebech, M.; Jensen, L.; Lind, P.; Rask, M.; Bagger, P.; Björkman, C.; Uggla, A. Neospora caninum infection and repeated abortions in humans. Emerg. Infect. Dis. 1999, 5, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Cobo, M.J.; Herrera-Viedma, E.; Herrera, F.; López-Herrera, A. SciMAT: A new science mapping analysis software tool. J. Am. Soc. Inf. Sci. Technol. 2012, 63, 1609–1630. [Google Scholar] [CrossRef]
- Cobo, M.-J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. An approach for detecting, quantifying, and visualizing the evolution of a research field: A practical application to the Fuzzy Sets Theory field. J. Inf. 2011, 5, 146–166. [Google Scholar] [CrossRef]






| Search Terms | MEDLINE | Dimensions 1 | Scopus 2 | WoS 3 | WoS 4 |
|---|---|---|---|---|---|
| Neospora | 2379 | 2905 | 2998 | 3068 | 3470 |
| (“Neospora”) AND Diagnosis | 1016 | 329 | 442 | 872 | 882 |
| (“Neospora”) AND pathology | 430 | 42 | 211 | 80 | 147 |
| (“Neospora”) AND pathogenesis | 2010 | 76 | 115 | 143 | 145 |
| Topic Number | Topic Words | Number of Publications Contributing to Topic Model | Lay Description of Topic |
|---|---|---|---|
| 25 | caninum, Neospora, cattle, neosporosis, parasite, infection, abortion, bovine, important, disease | 1541 | Neospora as a cause of abortion in cattle |
| 11 | caninum, antibodies, Neospora, seroprevalence, infection, samples, study, positive, prevalence, presence | 1199 | Seroprevalence of Neospora |
| 13 | development, disease, studies, species, including, animal, diseases, vaccine, approach, understanding | 1130 | Disease development and control |
| 49 | gondii, Toxoplasma, caninum, Neospora, parasites, infections, toxoplasmosis, animals, study, species | 911 | Neospora and Toxoplasma infections in animals |
| 61 | caninum, pcr, dna, samples, Neospora, positive, detection, detected, brain, tissues | 802 | PCR detection of Neospora in animal tissues |
| 97 | antibody, ifat, caninum, test, antibodies, indirect, Neospora, serum, fluorescent, samples | 757 | Immunofluorescent antibody testing |
| 29 | caninum, mice, infection, Neospora, tachyzoites, infected, days, inoculated, parasite, experimental | 709 | Experimental infections in mice |
| 27 | caninum, Neospora, lesions, infection, neosporosis, brain, tachyzoites, immunohistochemical, immunohistochemistry, encephalitis | 559 | Immunohistochemistry and pathology |
| 17 | cows, caninum, seropositive, dairy, Neospora, abortion, seronegative, heifers, animals, cattle | 540 | Serology in cattle |
| 36 | risk, factors, farms, seroprevalence, study, seropositivity, analysis, regression, herds, logistic | 512 | Risk analyses |
| 4 | cattle, prevalence, caninum, seroprevalence, herds, dairy, Neospora, infection, beef, herd | 505 | Seroprevalence of Neospora in cattle |
| 19 | test, tests, results, sensitivity, bovine, elisa, serological, sera, specificity, cattle | 489 | Serological diagnostics for cattle |
| 78 | caninum, antigens, antigen, kDa, tachyzoites, Neospora, recognized, tachyzoite, antibodies, monoclonal | 483 | Identification of antigens |
| 43 | levels, compared, study, significant, significantly, observed, results, higher, increased, found | 476 | Diagnostics for cattle |
| 33 | host, parasite, cell, Neospora, parasites, intracellular, caninum, infection, apicomplexan, Toxoplasma | 471 | Neospora and Toxoplasma infections in animals |
| Cluster | Cluster/Lay Description | Keywords | Species and Diseases | Countries Mentioned |
|---|---|---|---|---|
| 1 | Neospora caninum | Brain, calf, isolation, bradyzoite, detection, isolate, isolation, PCR, oocyst, molecular characterisation | Besnoitia, Hammondia, Hammondia heydorni, Sarcocystis | Japan |
| 2 | Mouse models | Experimental infection, gestation, immunisation, placenta, pregnancy, protection, strain, toltrazuril, vaccine, vertical transmission | Balb/C mouse | |
| 3 | Reproduction | Abortion, association, cause, evidence, herd, reproductive performance, review, risk, seroepidemiology, serological survey | Bubalus bubalis (water buffalo), dairy cow, Mycobacterium avium subspecies paratubuculosis, | Argentina, Australia, Iran, New Zealand |
| 4 | Seroepidemiology | Anti Neospora caninum antibody, blood, cat, control, diagnosis, elisa, epidemiology, milk, serodiagnosis, transplacental transmission | bovine neosporosis, Toxoplasma gondii infection, toxoplasmosis | |
| 5 | Seroprevalence | dairy farm, disease, investigation, Neospora caninum antibody, occurrence, population, risk factor, seroprevalence, serosurvey | Beef cattle, goat, sheep, Toxoplasma gondii | China, Mexico, Brazil, Spain, Turkey |
| 6 | Molecular detection in Brazil | Bovino, molecular detection, state | Neospora spp. | Brazilian locations |
| 7 | Treatment of cyst-forming coccidia | Antibody, apicomplexan, chapter, factor, protozoa, treatment | Equine protozoal myeloencephalitis, horse, Neospora, Toxoplasma, Sarcocystis | Italy |
| 8 | Foxes | Parasite, prevalence, Toxoplasma gondii antibody | Vulpes vulpes (red fox), | Czech republic, Germany |
| 9 | Prevalence in Columbia | Presence, seroprevalence | Columbia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichel, M.P.; Wahl, L.C.; Ellis, J.T. Research into Neospora caninum—What Have We Learnt in the Last Thirty Years? Pathogens 2020, 9, 505. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060505
Reichel MP, Wahl LC, Ellis JT. Research into Neospora caninum—What Have We Learnt in the Last Thirty Years? Pathogens. 2020; 9(6):505. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060505
Chicago/Turabian StyleReichel, Michael P., Lloyd C. Wahl, and John T. Ellis. 2020. "Research into Neospora caninum—What Have We Learnt in the Last Thirty Years?" Pathogens 9, no. 6: 505. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9060505