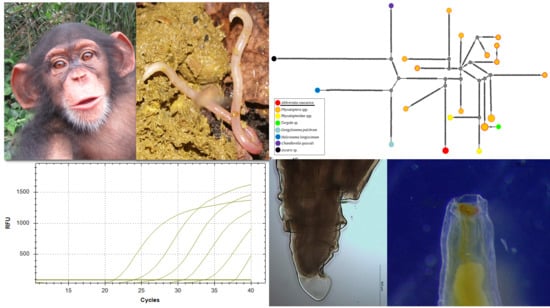
Zoonotic Abbreviata caucasica in Wild Chimpanzees (Pan troglodytes verus) from Senegal
Abstract
:1. Introduction
2. Results
2.1. Morphological Characteristics of Adult A. caucasica
2.2. A. caucasica Eggs from Positive Feces
2.3. Molecular Characterization of Adult A. caucasica Worms
2.4. Molecular Investigation of A. caucasica and Nematode Infestation from Biological Samples
2.5. The Analytical Sensitivity of A. caucasica 12S rRNA qPCR and Egg Counting
3. Discussion
4. Materials and Methods
4.1. Study Site and Study Subjects
4.2. Fecal, Worms, and Environmental Samples
4.3. Morphological Analysis of A. caucasica Adult Worms
4.4. Identification of A. caucasica Eggs from Positive Feces
4.5. DNA Extraction
4.6. Molecular Characterization of Adult Worms
4.6.1. Development of PCR Primer Sets
4.6.2. Polymerase Chain Reaction, Sequencing and Phylogenetic Analysis
4.7. TaqMan qPCR for Nematoda Parasites Detection
4.8. Quantitative TaqMan Real-Time PCR (qPCR) for A. caucasica Detection
4.9. Conventional PCR Specific for A. caucasica
4.10. Molecular Survey of A. caucasica and Nematode Infestations in a Chimpanzee Population and the Environmental Samples
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strait, K.; Else, J.G.; Eberhard, M.L. Parasitic Diseases of Nonhuman Primates, 2nd ed.; Elsevier Inc: Amsterdam, The Netherlands, 2012; ISBN 9780123813664. [Google Scholar]
- Cleeland, L.M.; Reichard, M.V.; Tito, R.Y.; Reinhard, K.J.; Lewis, C.M. Clarifying prehistoric parasitism from a complementary morphological and molecular approach. J. Archaeol. Sci. 2013, 40, 3060–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makki, M.; Dupouy-Camet, J.; Seyed Sajjadi, S.M.; Moravec, F.; Naddaf, S.R.; Mobedi, I.; Malekafzali, H.; Rezaeian, M.; Mohebali, M.; Kargar, F.; et al. Human spiruridiasis due to Physaloptera spp. (Nematoda: Physalopteridae) in a grave of the Shahr-e Sukhteh archeological site of the Bronze Age (2800–2500 BC) in Iran. Parasite 2017, 24, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, B.B. The Nematode genus Abbreviata (Travassos, 1920) Schulz, 1927. Am. Midl. Nat. 1945, 34, 485–490. [Google Scholar] [CrossRef]
- Poinar, G.O.; Quentin, J.-C. The development of Abbreviata caucasica (Von Linstow) (Spirurida: Physalopteridae) in an intermediate host. J. Parasitol. 1972, 58, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Metzger, S. Gastrointestinal helminthic parasites of habituated wild chimpanzees (Pan troglodytes verus) in the Tai NP, Cote d’Ivoire-including characterization of cultured helminth developmental stages using genetic markers. Ph.D. Thesis, Freie University, Berlin, Germany, 2014. [Google Scholar]
- Fain, A.; Vandepitte, J. Description des physaloptères (Abbreviata caucasica Linstow, 1902) récoltés chez l’homme au Congo. Bull. Acad. R. Med. Belg. 1964, 4, 663–682. [Google Scholar] [PubMed]
- Schulz, R.-E. Sur la morphologie du Physaloptera caucasica von Linstow, 1902, de l’homme. Ann. Parasitol. Hum. Comparée 1926, 4, 74–84. [Google Scholar] [CrossRef]
- Ortlepp, R.J. On the identity of Physaloptera caucasica v. Linstow, 1902, and Physaloptera mordens Leiper, 1908. J. Helminthol. 1926, 4, 199. [Google Scholar] [CrossRef]
- Calle, P.P.; Ott Joslin, J. New world and old world monkeys. In Fowler’s Zoo and Wild Animal Medicine; Miller, E., Fowler, M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 8, pp. 301–335. [Google Scholar]
- Mul, I.F.; Paembonan, W.; Singleton, I.; Wich, S.A.; Van Bolhuis, H.G. Intestinal parasites of free-ranging, semicaptive, and captive Pongo abelii in Sumatra, Indonesia. Int. J. Primatol. 2007, 28, 407–420. [Google Scholar] [CrossRef] [Green Version]
- McGrew, W.C.; Tutin, C.E.G.; Collins, D.A.; File, S.K. Intestinal parasites of sympatric Pan troglodytes and Papio spp. at two sites: Gombe (Tanzania) and Mt. Assirik (Senegal). Am. J. Primatol. 1989, 17, 147–155. [Google Scholar] [CrossRef]
- Gillespie, T.R.; Lonsdorf, E.V.; Canfield, E.P.; Meyer, D.J.; Nadler, Y.; Raphael, J.; Pusey, A.E.; Pond, J.; Pauley, J.; Mlengeya, T.; et al. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am. J. Phys. Anthropol. 2010, 143, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Howells, M.E.; Pruetz, J.; Gillespie, T.R. Patterns of gastro-intestinal parasites and commensals as an index of population and ecosystem health: The case of sympatric western chimpanzees (Pan troglodytes verus) and guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. Am. J. Primatol. 2011, 73, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K. Zoonoses: Infectious diseases transmissible from animals to humans. J. Clin. Pathol. 2004, 57, 1120. [Google Scholar] [CrossRef] [Green Version]
- Petri, L.H. Life cycle of Physaloptera rara Hall and Wigdor, 1918 (Nematoda: Spiruroidea) with the Cockroach, Blatella germanica, serving as the intermediate host. Trans. Kansas Acad. Sci. 1950, 63, 331–337. [Google Scholar] [CrossRef]
- Petri, L.H.; Ameel, D.J. Studies on the life cycle of Physaloptera rara Hall and Wigdor, 1918 and Physaloptera praeputialis Linstow, 1889. J. Parasitol. 1950, 36, 6. [Google Scholar]
- Olsen, J.L. Life cycle of Physaloptera rara Hall and Wigdor, 1918 (Nematoda: Physalopteroidea) of canids and felids in definitive, intermediate and paratenic hosts. Rev. Iber. Parasitol. 1980, 40, 489–525. [Google Scholar]
- Flynn, R.J. Parasites of Laboratory Animals; Iowa State University Press: Ames, IA, USA, 1973; p. 884. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Brede, H.D.; Burger, P.J. Physaloptera caucasica (=Abbreviata caucasica) in the South African baboon (Papio ursinus). Arb Paul Ehrlich Inst Georg Speyer Haus Ferdinand Blum Inst Frankf A M. 1977, 71, 119–122. [Google Scholar]
- Harras, S.F.; Elmahy, R.A. New record of Abbreviata leptosoma Gervais, 1848 (Spirurida: Physalopteridae) infection in two species of lizards in north and south Sinai, Egypt. Egypt. J. Zool. 2019, 72, 1–10. [Google Scholar]
- Chabaud, A.G. Essai de révision des Physaloptères parasites de reptiles. Ann. Parasitol. Hum. Comparée 1956. [Google Scholar] [CrossRef]
- Fortuner, R. Ratios and indexes in nematode taxonomy. Nematologica 1990, 36, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Vovlas, N.; Subbotin, S.A.; Troccoli, A.; Liébanas, G.; Castillo, P. Molecular phylogeny of the genus Rotylenchus (Nematoda, Tylenchida) and description of a new species. Zool. Scr. 2008, 37, 521–537. [Google Scholar] [CrossRef] [Green Version]
- Avó, A.P.; Daniell, T.J.; Neilson, R.; Oliveira, S.; Branco, J.; Adão, H. DNA barcoding and morphological identification of benthic nematodes assemblages of estuarine intertidal sediments: Advances in molecular tools for biodiversity assessment. Front. Mar. Sci. 2017, 4, 66. [Google Scholar] [CrossRef] [Green Version]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Meldal, B.H.M.; Debenham, N.J.; De Ley, P.; De Ley, I.T.; Vanfleteren, J.R.; Vierstraete, A.R.; Bert, W.; Borgonie, G.; Moens, T.; Tyler, P.A.; et al. An improved molecular phylogeny of the Nematoda with special emphasis on marine taxa. Mol. Phylogenet. Evol. 2007, 42, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Barbuto, M.; Bain, O.; Galimberti, A.; Uni, S.; Guerrero, R.; Ferté, H.; Bandi, C.; Martin, C.; Casiraghi, M. Integrated taxonomy: Traditional approach and DNA barcoding for the identification of filarioid worms and related parasites (Nematoda). Front. Zool. 2009, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, F.B.; Alves, P.V.; Rocha, B.M.; de Souza Lima, S.; Luque, J.L. A new Physaloptera (Nematoda: Physalopteridae) Parasite of Tupinambis merianae (Squamata: Teiidae) from Southeastern Brazil. J. Parasitol. 2012, 98, 1227–1235. [Google Scholar] [CrossRef]
- Kalyanasundaram, A.; Henry, C.; Brym, M.Z.; Kendall, R.J. Molecular identification of Physaloptera sp. from wild northern bobwhite (Colinus virginianus) in the Rolling Plains ecoregion of Texas. Parasitol. Res. 2018, 117, 2963–2969. [Google Scholar] [CrossRef]
- Sao Luiz, J.; Simoes, R.; Torres, E.; Barbosa, H.; Santos, J.; Giese, E.; Rocha, F.; Maldonado-Junior, A. A new species of Physaloptera (Nematoda: Physalopteridae) from Cerradomys subflavus (Rodentia: Sigmodontidae) in the Cerrado Biome, Brazil. Neotrop. Helminthol. 2015, 9, 301–312. [Google Scholar]
- Maldonado, A.; SimAes, R.O.; Luiz, J.S.; Costa-Neto, S.F.; Vilela, R.V. A new species of Physaloptera (Nematoda: Spirurida) from Proechimys gardneri (Rodentia: Echimyidae) from the Amazon rainforest and molecular phylogenetic analyses of the genus. J. Helminthol. 2019, 94, e68. [Google Scholar] [CrossRef]
- De Oliveira, M.C.; Lima, V.F.; Pinto, C.L.D.M.; da Silva, É.G.; Teles, D.A.; Ferreira-Silva, C.; Almeida, W.D.O. New record of Physaloptera sp. (Nematoda: Physalopteridae) parasitizing Philodryas nattereri (Ophidia: Dipsadidae) in Brazil. Herpetol. Notes 2019, 12, 1031–1034. [Google Scholar]
- Velarde-Aguilar, M.G.; Romero-Mayén, Á.R.; León-Règagnon, V. First report of the genus Physaloptera (Nematoda: Physalopteridae) in Lithobates montezumae (Anura: Ranidae) from Mexico. Rev. Mex. Biodivers. 2014, 85, 304–307. [Google Scholar] [CrossRef] [Green Version]
- Ederli, N.B.; Gallo, S.S.M.; Oliveira, L.C.; de Oliveira, F.C.R. Correction to: Description of a new species Physaloptera goytaca n. sp. (Nematoda, Physalopteridae) from Cerradomys goytaca Tavares, Pessôa & Gonçalves, 2011 (Rodentia, Cricetidae) from Brazil. Parasitol. Res. 2018, 117, 2757–2766. [Google Scholar] [PubMed]
- Leiper, B.Y.R.T. Observation on certain helminths of men. Trans. R. Soc. Trop. Med. Hyg. 1915, 6, 265–297. [Google Scholar] [CrossRef]
- Pouillevet, H.; Dibakou, S.-E.; Ngoubangoye, B.; Poirotte, C.; Charpentier, M.J.E. A comparative study of four methods for the detection of nematode eggs and large protozoan cysts in mandrill faecal material. Folia Primatol. 2017, 88, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.C.; Henzi, S.P. Environmental correlates of gastrointestinal parasitism in montane and lowland baboons in Natal, South Africa. Int. J. Primatol. 1993, 14, 623–635. [Google Scholar] [CrossRef]
- Anderson, R.C. Nematode parasites of vertebrates. In Their Development and Transmission, 2nd ed.; CABI Publishing: Wallingford, Oxon, UK, 2000; p. 650. [Google Scholar]
- Martin, J. Australian mammals: Biology and captive management. Austral. Ecol. 2005, 30, 126–129. [Google Scholar] [CrossRef]
- King, C.; Jones, H.I.; Tay, C.Y. Arthropod intermediate hosts of Abbreviata antarctica (Nematoda: Physalopteridae) in Australia. J. Parasitol. 2013, 99, 708–711. [Google Scholar] [CrossRef]
- McGrew, W.C. Chimpanzee Material Culture: Implications for Human Evolution; Cambridge University Press: Cambridge, UK, 1993; p. 277. [Google Scholar]
- Pacheco, L.; Fraixedas, S.; Fernández-Llamazares, Á.; Estela, N.; Mominee, R.; Guallar, F. Perspectives on sustainable resource conservation in community nature reserves: A case study from senegal. Sustainability 2012, 4, 3158–3179. [Google Scholar] [CrossRef] [Green Version]
- McGrew, W.C.; Baldwin, P.J.; Tutin, C.E.G. Chimpanzees in a hot, dry and open habitat: Mt. Assirik, Senegal, West Africa. J. Hum. Evol. 1981. [Google Scholar] [CrossRef]
- Nishida, T. The social group of wild chimpanzees in the Mahali Mountains. Primates 1968, 9, 167–224. [Google Scholar] [CrossRef] [Green Version]
- Rudicell, R.S.; Piel, A.K.; Stewart, F.; Moore, D.L.; Learn, G.H.; Li, Y.; Takehisa, J.; Pintea, L.; Shaw, G.M.; Moore, J.; et al. High Prevalence of Simian Immunodeficiency Virus Infection in a Community of Savanna Chimpanzees. J. Virol. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillespie, T.R. Noninvasive assessment of gastrointestinal parasite infections in free-ranging primates. Int. J. Primatol. 2006, 27, 1129–1143. [Google Scholar] [CrossRef] [Green Version]
- Jessee, M.T.; Schilling, P.W.; Stunkard, J.A. Identification of intestinal helminth eggs in old world primates. Lab. Anim. Care 1970, 20, 83–87. [Google Scholar] [PubMed]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Koressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Laidoudi, Y.; Davoust, B.; Varloud, M.; Niang, E.H.A.; Fenollar, F.; Mediannikov, O. Development of a multiplex qPCR-based approach for the diagnosis of Dirofilaria immitis, D. repens and Acanthocheilonema reconditum. Parasites Vectors 2020, 13, 319. [Google Scholar] [CrossRef]
- Laidoudi, Y.; Ringot, D.; Watier-grillot, S.; Davoust, B.; Mediannikov, O. A cardiac and subcutaneous canine dirofilariosis outbreak in a kennel in central France. Parasite 2019, 26, 72. [Google Scholar] [CrossRef]
- Song, H.; Buhay, J.E.; Whiting, M.F.; Crandall, K.A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc. Natl. Acad. Sci. USA 2008, 105, 13486–13491. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [Green Version]
- Zuckerkandl, E.; Pauling, L. Evolutionary divergence and convergence in proteins. Evolv. Genes Proteins 1965. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, I.; Lindner, D.; Bayer, M.; Husmeier, D.; Mcguire, G.; Marshall, D.F.; Wright, F. TOPALi v2: A rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 2009, 25, 126–127. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Waddell, P.J.; Steel, M.A. General time-reversible distances with unequal rates across sites: Mixing Y and inverse Gaussian distributions with invariant sites. Mol. Phylogenet. Evol. 1997, 8, 398–414. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Janke, A.; Waddell, P.J.; Westerman, M.; Takenaka, O.; Murata, S.; Okada, N.; Pääbo, S.; Hasegawa, M. Conflict among individual mitochondrial proteins in resolving the phylogeny of eutherian orders. J. Mol. Evol. 1998, 47, 307–322. [Google Scholar] [CrossRef]
- Rice, P.; Longden, I.; Bleasby, A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000, 16, 276–277. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [Green Version]
- Leigh, J.; Bryant, D.; Steel, M. PopART (Population Analysis with Reticulate Trees). 2015. Available online: http://popart.otago.ac.nz/index.shtml (accessed on 22 June 2020).
- Bamuhiiga, J.; Williams, S.A.; Fischer, P.; Büttner, D.W. Detection of the filarial parasite Mansonella streptocerca in skin biopsies by a nested polymerase chain reaction-based assay. Am. J. Trop. Med. Hyg. 2017, 58, 816–820. [Google Scholar]
- Huang, X.Y.; Hirsh, D. A second trans-spliced RNA leader sequence in the nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 1989, 86, 8640–8644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bio-Rad, L. Real-Time PCR Applications Guide; Bio-Rad Laboratories Inc.: Hercules, CA, USA, 2006; p. 41. [Google Scholar]








| Measures | This Study | Measurement of Fain and Vandepitte (1964) | Measurement of Linstow, 1902 | Measurement of Schulz (1926) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. caucasica | A. caucasica (Syn. P. mordens) | A. caucasica: Type Species | A. caucasica: Type Species | P. Mordens: Type Species | ||||||
| Chimpanzee (Senegal) | Men (Congo) | Men (caucasia) | Men (caucasia) | Men (Uganda) | ||||||
| Organ | Segment | CHS 11 | CHS 31 | N1 | N1 | Adult Female | N1 | N2 | N1 | N2 |
| Body | Length | 54.7 | 59.6 | 108 | 117 | 27 | 24.75 | 23.84 | 41 | 100 |
| Width | 2.08 | 2.13 | 2 | 3 | 1.14 | 1.18 | 1.12 | 1.8 | 2.8 | |
| Index a | 26.3 | 28 | 54 | 39 | 23.68 | 20.97 | 21.29 | 22.78 | 35.71 | |
| Nerve ring | From the anterior end | 0.43 | - | 0.7 | 0.78 | - | 0.454 | 0.454 | - | - |
| Esophagus (e) | Total length | 5.52 | 4.82 | 9 | 11 | - | 3.5 | 3.72 | - | - |
| Length muscolar (e) | 0.79 | 0.75 | 0.6 | 0.6 | - | 0.43 | 0.35 | - | - | |
| Width | 0.268 | 0.287 | - | - | - | - | - | |||
| Index b | 9.91 | 12.37 | 12 | 10.63 | - | 7 | 6.4 | 6.2 | 6.2 | |
| Cuticle | Width | 0.92–0.102 | 0.70–0.122 | - | - | - | - | - | - | - |
| Vulva | From the anterior end | 1.56 | - | 21 | 23 | - | 3.50 | 4.62 | - | - |
| Eggs (μm) | 37–41 × 28–32 | 36–39 × 28–31 | 60–65 × 45–55 | 57 × 39 | 57–62 × 42–45 | 45–49 × 32–34 | ||||
| Tail | Tail | 1.084 | - | 1.3 | 1.4 | 0.51 | 0.578 | 0.532 | - | - |
| Index c | 50.46 | - | 80 | 83 | 53 | 43 | 45 | 70 | 90 | |
| Tested Samples | Infestation Rate (%) by qPCRs | ||
|---|---|---|---|
| A. caucasica | Nematodes | ||
| Localities | |||
| Locality 1 | 3 | 0 | 33.3 |
| Locality 2 | 6 | 66.7 | 100 |
| Locality 3 | 39 | 53.8 | 82.1 |
| Total | 48 | 56.3 | 81.3 |
| Comparison by localities | Fisher test (p) | 0.148 | 0.052 |
| Fecal consistency | |||
| Fresh | 38 | 40.0 | 70.0 |
| Degraded | 10 | 55.3 | 84.2 |
| Fisher test (p) | // | 0.49 | 0.30 |
| Fecal Consistency | Number of Positive Samples | Quantification (Means Eggs/g) from Positive Samples | |
|---|---|---|---|
| Degraded | 4 | 0.2 | |
| Fresh | 21 * | 1.4 | |
| Statistics | One-way ANOVA | R2 | 0.032 |
| Pr > F | 0.403 | ||
| Primer Name | Sequences 5′-3′ | Target Gene | Size (bp) | Melting Tm | Elongation Time | Specificity | Ref. |
|---|---|---|---|---|---|---|---|
| Fwd-ITS-793 | TCGATGAAGAACGCAGCTA | ITS2 | 420–750 | 57 °C | 1’ | Pan-Nematoda | This study |
| Rwd-ITS-1495 | AGTTTCTTTTCCTCCGCTTAGTT | ||||||
| Fwd-12S-Nem-1 | AAGTTTGATTTTGGTTTTGGTTG | 12S | 680 | 58 °C | 1’ | ||
| Rwd-12S-Nem-681 | CCATTGACGGATGGTTTGTA | ||||||
| Fwd-16S-Nem-488 | GCAGCCTTAGCGTGATGG | 16S | 430 | 58 °C | 1’ | ||
| Rwd-16S-Nem-918 | TAAACCGCTCTGTCTCACGA | ||||||
| dg.Fwd.COI.Nem.257 | TTGGKGGTTTTGGWAATTGG | Cox 1 | 1069 | 52 °C | 1’30” | ||
| dg.Rwd.COI.Nem.1325 | CCAGCAAAATGCAWAGGAAAA | ||||||
| Fwd.18S.631 | TCGTCATTGCTGCGGTTAAA | 18S | 1127–1155 | 54 °C | 1’30” | Pan-Nematoda | [53] |
| Rwd.18S.1825 | GGTTCAAGCCACTGCGATTAA | ||||||
| Fwd.Abbrev.COI.51f | TGATCAGGGTTGGGAGCTT | Cox 1 | 550 | 53 °C | 1’ | A. caucasica | This study |
| Rwd.Abbrev.COI.601r | AAAAAGAACAATTAAAATTACGATCC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laidoudi, Y.; Medkour, H.; Latrofa, M.S.; Davoust, B.; Diatta, G.; Sokhna, C.; Barciela, A.; Hernandez-Aguilar, R.A.; Raoult, D.; Otranto, D.; et al. Zoonotic Abbreviata caucasica in Wild Chimpanzees (Pan troglodytes verus) from Senegal. Pathogens 2020, 9, 517. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9070517
Laidoudi Y, Medkour H, Latrofa MS, Davoust B, Diatta G, Sokhna C, Barciela A, Hernandez-Aguilar RA, Raoult D, Otranto D, et al. Zoonotic Abbreviata caucasica in Wild Chimpanzees (Pan troglodytes verus) from Senegal. Pathogens. 2020; 9(7):517. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9070517
Chicago/Turabian StyleLaidoudi, Younes, Hacène Medkour, Maria Stefania Latrofa, Bernard Davoust, Georges Diatta, Cheikh Sokhna, Amanda Barciela, R. Adriana Hernandez-Aguilar, Didier Raoult, Domenico Otranto, and et al. 2020. "Zoonotic Abbreviata caucasica in Wild Chimpanzees (Pan troglodytes verus) from Senegal" Pathogens 9, no. 7: 517. https://0-doi-org.brum.beds.ac.uk/10.3390/pathogens9070517