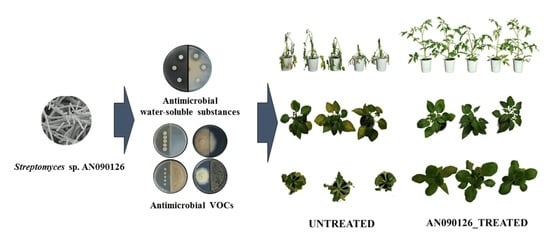
Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture and Fermentation Conditions
2.2. Phytopathogenic Bacteria and Fungi
2.3. Identification and Morphological and Biochemical Observations of the AN090126 Strain
2.4. Preparation of PPF
2.5. Minimum Inhibitory Concentration (MIC) Assay
2.6. Checkerboard Assay
FICB = (MIC of B in combination) ÷ (MIC of B alone)
FICI = FICA + FICB
2.7. Antimicrobial Bioassays of VOCs
2.8. Collection and Analysis of Volatile Organic Compound (VOC) Production Using Solid-Phase Microextraction (SPME)/Gas Chromatography-Mass Spectrometry (GC-MS)
2.9. Plant Materials
2.10. In Vivo Assay
2.11. Statistical Analysis
3. Results
3.1. Identification of Streptomyces sp. AN090126
3.2. Antimicrobial Activity of Streptomyces sp. AN090126
3.3. VOCs Produced by Streptomyces sp. AN090126 and Their Effects on Microbial Growth
3.4. Efficacy of the Fermentation Broth of Streptomyces sp. AN090126 in Controlling Plant Bacterial and Fungal Diseases
3.5. Interaction between PPF and Streptomycin Sulfate
3.5.1. In Vitro Experiment
3.5.2. In Vivo Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.R.; Anwer, M.A. Fungal bioinoculants for plant disease management. In Microbes and Microbial Technology: Agricultural and Environmental Applications; Ahmad, I., Ahmad, F., Pichtel, J., Eds.; Springer: New York, NY, USA, 2011; pp. 447–488. [Google Scholar]
- Elad, Y.; Pertot, I. Climate change impacts on plant pathogens and plant diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Lazarovits, G.; Turnbull, A.; Johnston-Monje, D. Plant Health Management: Biological Control of Plant Pathogens; Academic Press: Cambridge, MA, USA, 2014; pp. 388–399. [Google Scholar]
- Sundin, G.W.; Castiblanco, L.F.; Yuan, X.; Zeng, Q.; Yang, C.-H. Bacterial disease management: Challenges, experience, innovation and future prospects. Mol. Plant Pathol. 2016, 17, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Koutroubas, S.D. Current status and recent developments in biopesticide use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Mirik, M.; Aysan, Y.; Cinar, O. Biological control of bacterial spot disease of pepper with Bacillus strains. Turk. J. Agric. For. 2008, 32, 381–390. [Google Scholar]
- Powell, J.; Vargas, J., Jr.; Nair, M.; Detweiler, A.; Chandra, A. Management of dollar spot on creeping bentgrass with metabolites of Pseudomonas aureofaciens (TX-1). Plant Dis. 2000, 84, 19–24. [Google Scholar] [CrossRef]
- Newitt, J.T.; Prudence, S.M.M.; Hutchings, M.I.; Worsley, S.F. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Yang, S.H.; Palaniyandi, S.A.; Han, J.S.; Yoon, T.-M.; Kim, T.-J.; Suh, J.-W. Azalomycin F complex is an antifungal substance produced by Streptomyces malaysiensis MJM1968 isolated from agricultural soil. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 545–552. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Qiao, X.; Li, Z.; Li, F.; Chen, M.; Wang, Y.; Huang, Y.; Cui, H. Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiol. Lett. 2013, 341, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A.; Ramadan, G.A.; Elbadawi, A.A.; Hassan, A.H.; Tariq, S.; Ghazal, E.W.; Abo Gamar, M.I.; AbuQamar, S.F. The marine endophytic polyamine-producing Streptomyces mutabilis UAE1 isolated from extreme niches in the Arabian gulf promotes the performance of mangrove (Avicennia marina) seedlings under greenhouse conditions. Front. Mar. Sci. 2021, 8, 710200. [Google Scholar] [CrossRef]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.-H.I.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K.A. Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Le, K.D.; Kim, J.; Nguyen, H.T.; Yu, N.H.; Park, A.R.; Lee, C.W.; Kim, J.C. Streptomyces sp. JCK-6131 protects plants against bacterial and fungal diseases via two mechanisms. Front. Plant Sci. 2021, 12, 726266. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.-W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biot. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- Al Raish, S.M.; Saeed, E.E.; Alyafei, D.M.; El-Tarabily, K.A.; AbuQamar, S.F. Evaluation of streptomycete actinobacterial isolates as biocontrol agents against royal poinciana stem canker disease caused by the fungal pathogen Neoscytalidium dimidiatum. Biol. Control 2021, 164, 104783. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; AlKhajeh, A.S.; Ayyash, M.M.; Alnuaimi, L.H.; Sham, A.; ElBaghdady, K.Z.; Tariq, S.; AbuQamar, S.F. Growth Promotion of Salicornia bigelovii by Micromonospora chalcea UAE1, an endophytic 1-aminocyclopropane-1-carboxylic acid deaminase-producing actinobacterial isolate. Front. Microbiol. 2019, 10, 1694. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev. Sci. Tech. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Richardson, M.; Holt, J.N. Synergistic action of streptomycin with other antibiotics of intracellular Brucella abortus in vitro. J. Bacteriol. 1962, 84, 638–646. [Google Scholar] [CrossRef] [Green Version]
- El-Tarabily, K.A.; Sykes, M.L.; Kurtböke, I.D.; Hardy, G.E.S.J.; Barbosa, A.M.; Dekker, R.F.H. Synergistic effects of a cellulase-producing Micromonospora carbonacea and an antibiotic-producing Streptomyces violascens on the suppression of Phytophthora cinnamomi root rot of Banksia grandis. Can. J. Bot. 1996, 74, 618–624. [Google Scholar] [CrossRef] [Green Version]
- Challis, G.L.; Hopwood, D.A. Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. S2), 14555–14561. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.; Ouk Kim, Y.; Park, S.-C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef] [PubMed]
- Valan Arasu, M.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. In vitro antimicrobial activity of Streptomyces spp. ERI-3 isolated from Western Ghats rock soil (India). J. Mycol. Med. 2009, 19, 22–28. [Google Scholar] [CrossRef]
- Le, K.D.; Kim, J.; Yu, N.H.; Kim, B.; Lee, C.W.; Kim, J.C. Biological control of tomato bacterial wilt, Kimchi cabbage soft rot, and red pepper bacterial leaf spot using Paenibacillus elgii JCK-5075. Front. Plant Sci. 2020, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Le, K.D.; Yu, N.H.; Kim, J.I.; Kim, J.-C.; Lee, C.W. Structure and antifungal activity of pelgipeptins from Paenibacillus elgii against phytopathogenic fungi. Pestic. Biochem. Physiol. 2020, 163, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Kim, J.-C.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Choi, T.H.; Yoon, T.M.; Lee, S.-W. Effect of gallotannins derived from Sedum takesimense on tomato bacterial wilt. Plant Dis. 2013, 97, 1593–1598. [Google Scholar] [CrossRef] [Green Version]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Zang, H.; Wu, H.; Uddin Rajer, F.; Gao, X. Antibacterial effects of volatiles produced by Bacillus strain D13 against Xanthomonas oryzae pv. oryzae. Mol. Plant Pathol. 2018, 19, 49–58. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Extracellular proteases from Streptomyces phaeopurpureus ExPro138 inhibit spore adhesion, germination and appressorium formation in Colletotrichum coccodes. J. Appl. Microbiol. 2013, 115, 207–217. [Google Scholar] [CrossRef]
- Al Hamad, B.M.; Al Raish, S.M.; Ramadan, G.A.; Saeed, E.E.; Alameri, S.S.A.; Al Senaani, S.S.; AbuQamar, S.F.; El-Tarabily, K.A. Effectiveness of augmentative biological control of Streptomyces griseorubens UAE2 depends on 1-aminocyclopropane-1-carboxylic acid deaminase activity against Neoscytalidium dimidiatum. J. Fungi 2021, 7, 885. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Shao, H.; Zhang, K.; Li, G. Antibacterial metabolites from the actinomycete Streptomyces sp. P294. J. Microbiol. 2016, 54, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, S.; Zhang, J.; Wu, W. Antibacterial activity composition of the fermentation broth of Streptomyces djakartensis NW35. Molecules 2013, 18, 2763–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamil, F.H.; Saeed, E.E.; El-Tarabily, K.A.; AbuQamar, S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using streptomycete and non-streptomycete actinobacteria in the United Arab Emirates. Front. Microbiol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Saeed, E.E.; Sham, A.; Salmin, Z.; Abdelmowla, Y.; Iratni, R.; El-Tarabily, K.; AbuQamar, S. Streptomyces globosus UAE1, a potential effective biocontrol agent for black scorch disease in date palm plantations. Front. Microbiol. 2017, 8, 1455. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; ElBaghdady, K.Z.; AlKhajeh, A.S.; Ayyash, M.M.; Aljneibi, R.S.; El-Keblawy, A.; AbuQamar, S.F. Polyamine-producing actinobacteria enhance biomass production and seed yield in Salicornia bigelovii. Biol. Fert. Soils 2020, 56, 499–519. [Google Scholar] [CrossRef]
- Boukaew, S.; Plubrukam, A.; Prasertsan, P. Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solani on rice leaf. BioControl 2013, 58, 471–482. [Google Scholar] [CrossRef]
- Alblooshi, A.A.; Purayil, G.P.; Saeed, E.E.; Ramadan, G.A.; Tariq, S.; Altaee, A.S.; El-Tarabily, K.A.; AbuQamar, S.F. Biocontrol potential of endophytic actinobacteria against Fusarium solani, the causal agent of sudden decline syndrome on date palm in the UAE. J. Fungi 2022, 8, 8. [Google Scholar] [CrossRef]
- Cordovez, V.; Carrion, V.J.; Etalo, D.W.; Mumm, R.; Zhu, H.; van Wezel, G.P.; Raaijmakers, J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 2015, 6, 1081. [Google Scholar] [CrossRef] [Green Version]
- Dandurishvili, N.; Toklikishvili, N.; Ovadis, M.; Eliashvili, P.; Giorgobiani, N.; Keshelava, R.; Tediashvili, M.; Vainstein, A.; Khmel, I.; Szegedi, E.; et al. Broad-range antagonistic rhizobacteria Pseudomonas fluorescens and Serratia plymuthica suppress Agrobacterium crown gall tumours on tomato plants. J. Appl. Microbiol. 2011, 110, 341–352. [Google Scholar] [CrossRef]
- Ossowicki, A.; Jafra, S.; Garbeva, P. The antimicrobial volatile power of the rhizospheric isolate Pseudomonas donghuensis P482. PLoS ONE 2017, 12, e0174362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Hsiang, T. Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol. Technol. 2010, 58, 157–165. [Google Scholar] [CrossRef]
- Tyc, O.; Zweers, H.; de Boer, W.; Garbeva, P. Volatiles in inter-specific bacterial interactions. Front. Microbiol. 2015, 6, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morohoshi, T.; Shiono, T.; Takidouchi, K.; Kato, M.; Kato, N.; Kato, J.; Ikeda, T. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl. Environ. Microbiol. 2007, 73, 6339–6344. [Google Scholar] [CrossRef] [Green Version]
- Chernin, L.; Toklikishvili, N.; Ovadis, M.; Kim, S.; Ben-Ari, J.; Khmel, I.; Vainstein, A. Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 2011, 3, 698–704. [Google Scholar] [CrossRef]
- Nion, Y.A.; Toyota, K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 2015, 16, 907–920. [Google Scholar] [CrossRef]
- Shoda, M. Bacterial control of plant diseases. J. Biosci. Bioeng. 2000, 89, 515–521. [Google Scholar] [CrossRef]
- Hassan, E.O.; Zyton, M.A. Management of bacterial spot of pepper caused by Xanthomonas campestris pv. vesicatoria. Am. J. Biosci. Bioeng. 2017, 5, 41–49. [Google Scholar]
- Wei, Z.; Yang, X.; Yin, S.; Shen, Q.; Ran, W.; Xu, Y. Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl. Soil Ecol. 2011, 48, 152–159. [Google Scholar] [CrossRef]
- Ling, L.; Han, X.; Li, X.; Zhang, X.; Wang, H.; Zhang, L.; Cao, P.; Wu, Y.; Wang, X.; Zhao, J.; et al. A Streptomyces sp. NEAU-HV9: Isolation, identification, and potential as a biocontrol agent against Ralstonia solanacearum of tomato plants. Microorganisms 2020, 8, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Boland, G.J. Suppression of dollar spot by hypovirulent isolates of Sclerotinia homoeocarpa. Phytopathology 1998, 88, 788–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, E.B.; Craft, C. Introduction and establishment of strains of Enterobacter cloacae in golf course turf for the biological control of dollar spot. J. Plant Dis. 1991, 75, 510–514. [Google Scholar] [CrossRef]
- Springer, B.; Kidan, Y.G.; Prammananan, T.; Ellrott, K.; Böttger, E.C.; Sander, P. Mechanisms of streptomycin resistance: Selection of mutations in the 16S rRNA gene conferring resistance. Antimicrob. Agents Chemother. 2001, 45, 2877–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maryam, L.; Khan, A.U. A mechanism of synergistic effect of streptomycin and cefotaxime on CTX-M-15 type β-lactamase producing strain of E. cloacae: A first report. Front. Microbiol. 2016, 7, 2007. [Google Scholar] [CrossRef] [PubMed]



| Plant Pathogenic Bacteria | Minimum Inhibitory Concentration 1 | |||
|---|---|---|---|---|
| FF, % | PPF, µg/mL | Ss 2, µg/mL | Oa 3, µg/mL | |
| Acidovorax avenae subsp. cattleyae | 0.63 ± 0.00 d | 20.82 ± 9.04 e | 500 ± 0.00 b | 0.07 ± 0.03 f |
| Acidovorax konjaci | 0.83 ± 0.36 d | 20.82 ± 9.04 e | 7.81± 0.00 e | 0.78 ± 0.00 de |
| Agrobacterium tumefaciens | 10.00 ± 0.00 a | 250.00 ± 0.00 a | 166.67 ± 72.17 c | 1.04 ± 0.45 cd |
| Burkholderia glumae | 10.00 ± 0.00 a | 250.00 ± 0.00 a | 20.84 ± 9.02 e | 0.1 ± 0.00 f |
| Clavibacter michiganensis subsp. michiganensis | 8.33 ± 2.29 a | 250.00 ± 0.00 a | 20.84 ± 9.02 e | 0.13 ± 0.06 f |
| Ewinia amylovora | 3.33 ± 1.44 bc | 62.50 ± 0.00 cd | 5.21 ± 2.25 e | 2.08 ± 0.91 ab |
| Pectobacterium carotovorum subsp. carotovorum | 2.50 ± 0.00 a | 52.08 ± 18.04 d | 15.63 ± 0.00 a | 1.56 ± 0.00 bc |
| Pectobacterium chrysanthemi | 3.33 ± 1.44 bc | 83.33 ± 36.08 c | 500 ± 0.00 b | 0.1± 0.00 f |
| Pseudomonas syringae pv. actinidiae | 2.50 ± 0.00 bc | 62.5 ± 0.00 cd | 15.63 ± 0.00 e | 2.61 ± 0.91 a |
| Pseudomonas syringae pv. lachrymans | 5.00 ± 0.00 b | 125.00 ± 0.00 b | 15.63 ± 0.00 e | 1.56± 0.00 bc |
| Ralstonia solanacearum | 1.67 ± 0.72 cd | 41.67 ± 18.04 de | 10.42 ± 4.52 e | 0.20 ± 0.00 ef |
| Sr-Pcc 4 | 2.50 ± 0.00 cd | 62.5± 0.00 cd | 1000 ± 0.00 a | 1.56± 0.00 bc |
| Xanthomonas arboricola pv. pruni | 2.50 ± 0.00 cd | 62.5 ± 0.00 cd | 20.83 ± 9.02 e | 0.39 ± 0.00 ef |
| Xanthomonas axonopodis pv. citri | 3.33 ± 1.44 bc | 62.5 ± 0.00 cd | 62.5 ± 0.00 d | 0.78 ± 0.00 de |
| Xanthomonas euvesicatoria | 2.08 ± 0.72 cd | 52.08 ± 18.04 d | 31.25 ± 0.00 e | 0.13 ± 0.06 f |
| Xanthomonas oryzae pv. oryzae | 1.25 ± 0.15 cd | 41.67 ± 18.04 de | 10.42 ± 4.52 e | 0.78 ± 0.00 de |
| Plant Pathogenic Fungi | Minimum Inhibitory Concentration 1 | |
|---|---|---|
| FF (%) | PPF (µg/mL) | |
| Botrytis cinerea | 0.63 ± 0.00 c | 15.6 ± 0.00 d |
| Colletotrichum coccodes | 3.33 ±1.44 a | 83.33 ± 36.08 a |
| Cryphonectria parasitica | 1.67 ± 0.72 bc | 31.25 ± 0.00 cd |
| Fusarium graminearum | 1.25 ± 0.00 bc | 31.25 ± 0.00 cd |
| Fusarium oxysporum f. sp. lycopersici | 1.67 ± 0.72 bc | 52.08 ± 18.04 bc |
| Magnaporthe oryzae | >10 | >500 |
| Phytophthora cactorum | 1.25 ± 0.00 bc | 31.25 ± 0.00 cd |
| Raffaelea quercus-mongolicae | 2.08 ± 0.72 b | 52.08 ± 18.04 bc |
| Rhizoctonia solani | 1.04 ± 0.36 bc | 26.03 ± 9.04 cd |
| Sclerotinia homoeocarpa | 2.08 ± 0.72 b | 62.5 ± 0.00 ab |
| Putative VOC | Retention Time, min | Peak Area, % 1 |
|---|---|---|
| 2,4-Dimethyl hexanoic acid, methyl ester | 2.94 | 6.16 ± 0.15 e |
| 3-Methyl-1-butanol | 3.22 | 28.41 ± 0.52 a |
| 2-Methyl-1-butanol | 3.27 | 17.24 ± 0.73 b |
| Dimethyl disulfide | 3.37 | 9.91 ± 0.37 d |
| 1-Methoxy-2-methyl benzene | 3.68 | 2.94 ± 0.09 hi |
| 1-Hexanol | 5.27 | 4.45 ± 0.13 f |
| 1,4-Dimethyl benzene | 5.71 | 2.73 ± 0.16 i |
| Dimethyl trisulfide | 7.12 | 3.22 ± 0.32 hi |
| 2-Propyl furan | 7.34 | 15.17 ± 0.16 c |
| 2-Ethyl-1-hexanol | 8.06 | 3.40 ± 0.16 g |
| 1-Octanol | 8.78 | 6.36 ± 0.13 e |
| Bacteria | FIC 1 | FICI 2 | Outcome | |
|---|---|---|---|---|
| Ss | PPF | |||
| Aac 3 | 0.25 ± 0.00 b | 0.42 ± 0.14 a | 0.67 ± 0.14 ab | Partial synergy |
| At | 0.50 ± 0.00 a | 0.50 ± 0.00 a | 1.00 ± 0.00 a | Additive |
| Pc | 0.17 ± 0.07 c | 0.41 ± 0.14 a | 0.58 ± 0.19 b | Partial synergy |
| Sr-Pcc | 0.25 ± 0.00 b | 0.42 ± 0.14 a | 0.67 ± 0.07 ab | Partial synergy |
| Xac | 0.25 ± 0.00 b | 0.50 ± 0.00 a | 0.75 ± 0.00 ab | Partial synergy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, K.D.; Yu, N.H.; Park, A.R.; Park, D.-J.; Kim, C.-J.; Kim, J.-C. Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases. Microorganisms 2022, 10, 791. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms10040791
Le KD, Yu NH, Park AR, Park D-J, Kim C-J, Kim J-C. Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases. Microorganisms. 2022; 10(4):791. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms10040791
Chicago/Turabian StyleLe, Khanh Duy, Nan Hee Yu, Ae Ran Park, Dong-Jin Park, Chang-Jin Kim, and Jin-Cheol Kim. 2022. "Streptomyces sp. AN090126 as a Biocontrol Agent against Bacterial and Fungal Plant Diseases" Microorganisms 10, no. 4: 791. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms10040791