Probiotics and Colon Cancer
Abstract
:1. Introduction
2. Colon Cancer and Probiotics in an Animal Model
3. Microorganisms Responsible for or Protective of Colon Cancer
4. Probiotics in Colon Cancer Prevention and Treatment
5. Probiotics as Adjuvant in Adverse Events
6. Conclusions
Funding
Conflicts of Interest
References
- Grace, C. The Role of the Gut Microbiome in Colorectal Cancer. Clin. Colon Rectal Surg. 2018, 31, 192–198. [Google Scholar]
- Farhana, L.; Banerjee, H.N.; Verma, M.; Majumdar, A.P.N. Role of Microbiome in Carcinogenesis Process and Epigenetic Regulation of Colorectal Cancer. Methods Mol. Biol. 2018, 1856, 35–55. [Google Scholar]
- Castellarin, M.; Warren, R.; Freeman, J.D.; Dreolini, L.; Krzywinski, M.; Strauss, J.; Barnes, R.; Watson, P.; Allen-Vercoe, E.; Moore, R.A.; et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012, 22, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Sinha, R.; Pei, Z.; Dominianni, C.; Wu, J.; Shi, J.; Goedert, J.J.; Hayes, R.B.; Yang, L. Human gut microbiome and risk for colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Mima, K.; Cao, Y.; Chan, A.T.; Qian, Z.R.; Nowak, J.A.; Masugi, Y.; Shi, Y.; Song, M.; da Silva, A.; Gu, M.; et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clin. Transl. Gastroenterol. 2016, 7, e200. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Bouley, C.; Boutron-Ruault, M.-C.; Cummings, J.H.; Franck, A.; Gibson, G.R.; Isolauri, E.; Moreau, M.-C.; Roberfroid, M.; Rowland, I. Functional food science and gastrointestinal physiology and function. Br. J. Nutr. 1998, 80 (Suppl. 1), S147–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization and World Health Organization Expert Consultation. Evaluation of Health and Nutritional Properties of Powder Milk and Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations and World Health Organization: Córdoba, Argentina, 2001; Available online: ftp://ftp.fao.org/es/esn/food/probio_report_en.pdf (accessed on 8 September 2005).
- Toscano, M.; De Grandi, R.; Pastorelli, L.; Vecchi, M.; Drago, L. A consumer’s guide for probiotics: 10 golden rules for a correct use. Dig. Liver Dis. 2017, 49, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. MBio 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; Housseau, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Ganapathy, V.; Thangaraju, M.; Prasad, P.D.; Martin, P.M.; Singh, N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Curr. Opin. Pharmacol. 2013, 13, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Wang, K.; Mucida, D.; Stewart, C.A.; Schnabl, B.; Jauch, D.; Taniguchi, K.; Yu, G.Y.; Osterreicher, C.H.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Ganesh, B.P.; Shi, Z.; Rasik Shah, R.; Fultz, R.; Major, A.; Venable, S.; Lugo, M.; Hoch, K.; Chen, X.; et al. Gut Microbe–Mediated Suppression of Inflammation-Associated Colon Carcinogenesis by Luminal Histamine Production. Am. J. Pathol. 2017, 187, 2323–2336. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Shim, Y.Y.; Cha, S.K.; Reaney, M.J.T.; Chee, K.M. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J. Med. Microbiol. 2012, 61, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, C.; Ge, S.; Zhang, M.; Jiang, L.; Cui, J.; Ren, F. Lactobacillus salivarius Ren prevent the early colorectal carcinogenesis in 1,2-dimethylhydrazine-induced rat model. J. Appl. Microbiol. 2014, 117, 208–216. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, X.; Fang, B.; Zhu, C.; Zhu, J.; Ren, F. Effects of Lactobacillus salivarius Ren on cancer prevention and intestinal microbiota in 1, 2-dimethylhydrazine-induced rat model. J. Microbiol. 2015, 53, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Kim, H.; Lee, K.-W.; Park, K.-Y. Dead nano-sized Lactobacillus plantarum inhibits azoxymethane/dextran sulfatesodium–induced colon cancer in Balb/c mice. J. Med. Food 2015, 18, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.-A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, W.C.; Kong, M.S.; Shi, H.N.; Walker, W.A.; Lin, C.Y.; Huang, C.T.; Lin, Y.C.; Jung, S.M.; Lin, T.Y. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Zackular, J.P.; Chen, G.Y.; Schloss, P.D. Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Evrard, B.; Coudeyras, S.; Dosgilbert, A.; Charbonnel, N.; Alamé, J.; Tridon, A.; Forestier, C. Dose-dependent immunomodulation of human dendritic cells by the probiotic Lactobacillus rhamnosus Lcr35. PLoS ONE 2011, 6, e18735. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, J.; Liu, Y.; Yue, W.; Luo, X. Toll-like receptor 2 monoclonal antibody or/and Toll-like receptor 4 monoclonal antibody increase counts of Lactobacilli and Bifidobacteria in dextran sulfate sodium-induced colitis in mice. J. Gastroenterol. Hepatol. 2012, 27, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Ghoneum, M.; Felo, N. Selective induction of apoptosis in human gastric cancer cells by Lactobacillus kefiri (PFT), a novel kefir product. Oncol. Rep. 2015, 34, 1659–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- del Carmen, S.; deMoreno de LeBlanc, A.; Levit, R.; Azevedo, V.; Langella, P.; Bermúdez-Humarán, L.G.; Guy LeBlanc, J. Anti-cancer effect of lactic acid bacteria expressing antioxidant enzymes or IL-10 in a colorectal cancer mouse model. Int. Immunopharm. 2017, 42, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shukla, G. Probiotics Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Sprague Dawley rats. Nutr. Cancer 2013, 65, 84–91. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumor Biol. 2013, 34, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Ewaschuk, J.B.; Walker, J.W.; Diaz, H.; Madsen, K.L. Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. 2006, 136, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, D.; Rashmi, B.S. Anti-cancer properties of probiotics: A natural strategy for cancer prevention. EC Nutr. 2016, 5, 1191–1202. [Google Scholar]
- Hatakka, K.; Holma, R.; El-Nezami, H.; Suomalainen, T.; Kuisma, M.; Saxelin, M.; Poussa, T.; Mykkänen, H.; Korpela, R. The influence of Lactobacillus rhamnosus LC705 together with Propionibacterium freudenreichii ssp. shermanii JS on potentially arcinogenic bacterial activity in human colon. Int. J. Food Microbiol. 2008, 128, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterology 2010, 57, 1411–1415. [Google Scholar] [PubMed]
- Liu, Z.; Qin, H.; Yang, Z.; Xia, Y.; Liu, W.; Yang, J.; Jiang, Y.; Zhang, H.; Yang, Z.; Wang, Y.; et al. Randomised clinical trial: The effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery: A double-blind study. Aliment Pharmacol. Ther. 2011, 33, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effects of the oral administration of the exopolysaccharide produced by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Galdeano, C.M.; Perdigón, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 2007, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L. Enhancement of epithelial barrier function by probiotics. J. Epithel. Biol. Pharmacol. 2012, 5, 55–59. [Google Scholar] [CrossRef]
- Karczewski, J.; Troost, F.J.; Konings, I.; Dekker, J.; Kleerebezem, M.; Brummer, R.J.M.; Wells, J.M. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 289, 851–859. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.M.; Perdigón, G. Yogurt feeding inhibits promotion and progression of experimental colorectal cancer. Med. Sci. Monit. 2004, 10, 96–104. [Google Scholar]
- Wan, Y.; Xin, Y.; Zhang, C.; Wu, D.; Ding, D.; Tang, L.; Owusu, L.; Bai, J.; Li, W. Fermentation supernatants of Lactobacillus delbrueckii inhibit growth of human colon cancer cells and induce apoptosis through a caspase 3–dependent pathway. Oncol. Lett. 2014, 7, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Rafter, J.; Bennett, M.; Caderni, G.; Clune, Y.; Hughes, R.; Karlsson, P.C.; Klinder, A.; O’Riordan, M.; O’Sullivan, G.C.; Pool-Zobel, P.; et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am. J. Clin. Nutr. 2007, 85, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A four-probiotics regimen reduces postoperative complications after colorectal surgery: A randomized, double-blind, placebo-controlled study. World J. Surg. 2015, 39, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt consumption and risk of colorectal cancer in the Italian European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef] [Green Version]
- Koliarakis, I.; Psaroulaki, A.; Nikolouzakis, T.K.; Sgantzos, M.N.; Goulielmos, G.; Androutsopoulos, V.P.; Tsiaoussis, J.; Tsatsakis, A.; Kokkinakis, M. Intestinal microbiota and colorectal cancer: A new aspect of research. J. BUON 2018, 23, 1216–1234. [Google Scholar] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Krebs, B. Prebiotic and synbiotic treatment before colorectal surgery–randomised double blind trial. Coll. Antropol. 2016, 40, 35–40. [Google Scholar] [PubMed]
- Della, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912–915. [Google Scholar] [CrossRef]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bysticky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.-Y.; Chan, W.-T.; Jiang, C.-B.; Cheng, M.-L.; Liu, C.-Y.; Chiau, J.-S.C.; Lee, H.-C. Amelioration of chemotherapy-induced intestinal mucositis by orally administered probiotics in a mouse model. PLoS ONE 2015, 10, e0141402. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.M.; Stringer, A.M.; Gibson, R.J.; Yeoh, A.S.J.; Hannam, S.; Keefe, D.M.K. VSL#3 probiotic treatment reduces chemotherapy-induced diarrhea and weight loss. Cancer Biol. Ther. 2007, 6, 1449–1454. [Google Scholar] [PubMed]
- Osterlund, P.; Ruotsalainen, T.; Korpela, R.; Ollus, M.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: Arandomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, L.; Guido, A.; Eusebi, L.H.; Laterza, L.; Grilli, D.; Cennamo, V.; Ceroni, L.; Barbieri, E.; Bazzoil, F. Effects of probiotics for the prevention and treatment of radiation-induced diarrhea. J. Clin. Gastroenterol. 2009, 43, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Packey, C.D.; Ciorba, M.A. Microbial influences on the small intestinal response to radiation injury. Curr. Opin. Gastroenterol. 2010, 26, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahara, T.; Yamamoto, E.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.O.; Sugai, T.; An, B.; et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014, 74, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Cancer Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Szkaradkiewicz, A.K. Characteristic of bacteriocines and their application. Pol. J. Microbiol. 2013, 62, 223–235. [Google Scholar] [PubMed]

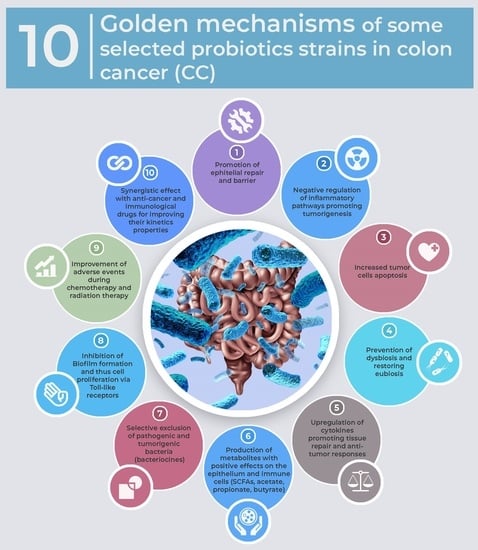
| Effect | Model Study | Listed Probiotics in CC Studies |
|---|---|---|
| Promotion of epithelial repair and barrier | Animal/Human [17,29,34,35,42,43] | Lactobacillus acidophilus Lactobacillus salivarius Lactobacillus plantarum Lactobacillus rhamnosus Lactobacillus kefiri Lactobacillus casei Lactobacillus delbrueckii Bifidobacterium infantis Bifidobacterium breve Bifidobacterium longum Streptococcus thermophilus |
| Negative regulation of inflammatory pathways promoting tumorigenesis | Animal/Human [9,10,27,28,33,36,41,42] | |
| Increased tumor cells apoptosis | Animal/Human [12,29,31,45] | |
| Prevention of dysbiosis and restoring eubiosis | Animal/Human [8,38,39,40,50] | |
| Upregulation of cytokines promoting tissue repair and antitumor responses | Animal/Human [16,44,46] | |
| Production of metabolites with positive effects on the epithelium and immune cells (SCFAs, acetate, propionate, butyrate) | Animal/Human [13,14,17,37] | |
| Selective exclusion of pathogenic and tumorigenic bacteria (bacteriocines) | Animal/Human [18,39,62] | |
| Inhibition of Biofilm formation and thus cell proliferation via Toll-like receptors | In vitro/Animal [28,62] | |
| Improvement of adverse events during chemotherapy and radiation therapy | Human [53,55,56,57,58,59] | |
| Synergistic effect with anti-cancer and immunological drugs for improving their kinetics properties | Hypothesis/Studies in progress [50,51] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7030066
Drago L. Probiotics and Colon Cancer. Microorganisms. 2019; 7(3):66. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7030066
Chicago/Turabian StyleDrago, Lorenzo. 2019. "Probiotics and Colon Cancer" Microorganisms 7, no. 3: 66. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7030066