Multiplexed High-Throughput Serological Assay for Human Enteroviruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Purification of Enterovirus Antigens
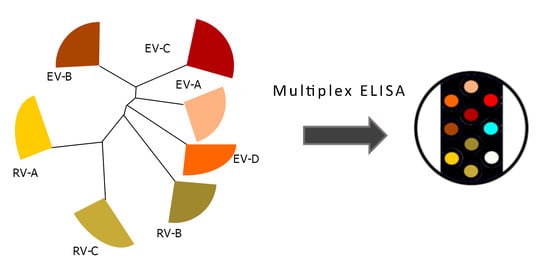
2.2. Setting Up the Multiplex Assay
2.3. Human Serum Samples
2.4. Human Samples Ethics Statement
2.5. Workflow for Antigen Preparation and Assay Run
2.6. Analysis of Results
3. Results
3.1. VP1 Antigens Belonging to All Human Enterovirus and Rhinovirus Species Were Produced in E. coli and Characterized
3.2. Antibody Responses to VP1 Antigens Are Stronger in Acutely Infected Adults Than in Healthy Controls
3.3. Antibody Responses to VP1 Antigens Are More Specific in Children Than in Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Serotype | Genbank | MW (kDa) | PI | Lysines |
|---|---|---|---|---|---|
| EV-A | CVA4 | AY421762.1 | 61.5 | 7.0 | 30 |
| EV-B | CVB1 | AB646478.11 | 58.9 | 8.65 | 33 |
| EV-C | PV1 | V01150.1 | 60.9 | 7.3 | 37 |
| EV-D | EV-D68 | EF107098.1 | 61.7 | 7.78 | 38 |
| RV-A | RV-A89 | M16248.1 | 61.7 | 7.68 | 38 |
| RV-B | RV-B14 | AY355195.1 | 59.9 | 6.52 | 40 |
| RV-C | RV-C3 | EF186077.2 | 58.2 | 6.23 | 36 |
| Antigen | Sequence |
|---|---|
| CVA4 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSGDAIADAIQNTVTSTIQRVTTNTVGQDATAANTAPSSHSLNTGLVPALQAAETGASSTATDGNLIETRCVVNSNGTRETHIEHFFSRSGLVGVMEVDDTGTSGKGFSNWDIDIMAFVQLRRKLEAFTYMRFDAEFTFVTNLENGLTNNSVIQYMYVPPGAPKPDARESFQWQTATNPSVFQKMDSPPPQVSVPFMSPASAYQWFYDGYPTFGPHSETSNLSYGQCPNNMLGTFSARVVSKQITNQKFQIRIYLRLKRVRAWIPRPLRSQPYIYRNYPTYGTTIQYLAKDRRKITETDYNAEQR THPGHHHHHHPG |
| CVB1 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSGPVEESVERAMVRVADTVSSKPTNSESIPALTAAETGHTSQVVPSDTMQTRHVKNYHSRSESSIENFLCRSACVYYATYTNNSKKGYAEWVINTRQVAQLRRKLELFTYLRFDLELTFVITSAQQPSTATSVDAPVQTHQIMYVPPGGPVPTKVTDYAWQTSTNPSVFWTEGNAPPRMSIPFISIGNAYSCFYDGWTQFSRNGVYGINTLNNMGTLYMRHVNEAGQGPIKSTVRIYFKPKHVKAWVPRPP RLCQYEKQKNVNFNPTGVTTTRSNITTTGAFPGHHHHHHPG |
| PV1 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSGLGQMLESMIDNTVRETVGAATSRDALPNTEASGPAHSKEIPALTAVETGATNPLVPSDTVQTRHVVQHRSRSESSIESFFARGACVAIITVDNSASTKNKDKLFTVWKITYKDTVQLRRKLEFFTYSRFDMEFTFVVTANFTETNNGHALNQVYQIMYVPPGAPVPEKWDDYTWQTSSNPSIFYTYGTAPARISVPYVGISNAYSHFYDGFSKVPLKDQSAALGDSLYGAASLNDFGILAVRVVNDHNPTKVTSKIRVYLKPKHIRVWCPRPPRAVAYYGPGVDYKDGTLTPLSTKDLTTYP GHHHHHHPG |
| EV-D68 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSLDHLDAAEAAYQIESIIKTATDTVKSEISAELGVVPSLNAVETGASSNTEPEEAIQTRTVINQHGVSETLVENFLSRAALVSKRSFEYKNHTSSKARTDKNFFKWTINTKSFVQLRRKLELFTYLRFDAEITILTTVAVNGSSNSTYMGLPDLTLQAMFVPTGALTPEKQDSFHWQSGSNASVFFKISDPPARMTIPFMCINSAYSVFYDGFAGFEKSGLYGINPADTIGNLCVRIVNEHQPIGFTVTVRVYMKPKHIKAWAPRPPRTLPYMSIANANYRGKDRAPNALNAIIGNRESVKTMPHNI VTTPGHHHHHHPG |
| RV-A89 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSLDHLDAAEAAYQIESIIKTATDTVKSEISAELGVVPSLNAVETGASSNTEPEEAIQTRTVINQHGVSETLVENFLSRAALVSKRSFEYKNHTSSKARTDKNFFKWTINTKSFVQLRRKLELFTYLRFDAEITILTTVAVNGSSNSTYMGLPDLTLQAMFVPTGALTPEKQDSFHWQSGSNASVFFKISDPPARMTIPFMCINSAYSVFYDGFAGFEKSGLYGINPADTIGNLCVRIVNEHQPIGFTVTVRVYMKPKHIKAWAPRPPRTLPYMSIANANYRGKDRAPNALNAIIGNRESVKTMPHNI VTTPGHHHHHHPG |
| RV-B14 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSGLGDELEEVIVEKTKQTVASISSGPKHTQKVPILTANETGATMPVLPSDSIETRTTYMHFNGSETDVECFLGRAACVHVTEIQNKDATGIDNHREAKLFNDWKINLSSLVQLRKKLELFTYVRFDSEYTILATASQPDSANYSSNLVVQAMYVPPGAPNPKEWDDYTWQSASNPSVFFKVGDTSRFSVPYVGLASAYNCFYDGYSHDDAETQYGITVLNHMGSMAFRIVNEHDEHKTLVKIRVYHRAKHV EAWIPRAPRALPYTSIGRTNYPKNTEPVIKKRKGDIKSYPGHHHHHHPG |
| RV-C3 VP1 | MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLVPRGSNPVEEFVEHTLKEVLVVPDTQASGPVHTTKPQALGAVEIGATADVGPETLIETRYVMNDNTNAEAAVENFLGRSALWANLRLDQGFRKWEINFQEHAQVRKKFEMFTYVRFDLEITIVTNNKGLMQIMFVPPGITPPGGKDGREWDTASNPSVFFQPNSGFPRFTIPFTGLGSAYYMFYDGYDGTDDANINYGISLTNDMGTLCFRALDGTGASDIKVFGKPKHITAWIPRPPRATQYLHKFSTNYNKPK TSGSTELEPKHFFKYRQDITSITNLPGHHHHHHPG |

References
- Ahn, J.; Joo, C.H.; Seo, I.; Kim, D.; Kim, Y.K.; Lee, H. All CVB serotypes and clinical isolates induce irreversible cytopathic effects in primary cardiomyocytes. J. Med Virol. 2004, 75, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.; Okonko, I.; Adu, F. Comparative study of molecular and antigenic characterization for intratypic differentiation (ITD) of poliovirus strains. J. Med. Virol. 2012, 84, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Ramsingh, A.I. Coxsackievirus-Induced Pancreatitis. Viral Immunol. 2004, 17, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, X.; Qian, B.; Wu, G.; He, T.; Feng, J.; Gao, C.; Wang, L.; Wang, J.; Li, X.; et al. Characterization of the antibody response against EV71 capsid proteins in Chinese individuals by NEIBM-ELISA. Sci. Rep. 2015, 5, 10636. [Google Scholar] [CrossRef] [Green Version]
- Samuelson, A.; Forsgren, M.; Johansson, B.; Wahren, B.; Sällberg, M. Molecular basis for serological cross-reactivity between enteroviruses. Clin. Diagn. Lab. Immunol. 1994, 1, 336–341. [Google Scholar] [CrossRef]
- Kupila, L.; Vuorinen, T.; Vainionpää, R.; Marttila, R.J.; Kotilainen, P. Diagnosis of Enteroviral Meningitis by Use of Polymerase Chain Reaction of Cerebrospinal Fluid, Stool, and Serum Specimens. Clin. Infect. Dis. 2005, 40, 982–987. [Google Scholar] [CrossRef]
- Stene, L.C.; Rewers, M. Immunology in the clinic review series; focus on type 1 diabetes and viruses: The enterovirus link to type 1 diabetes: Critical review of human studies. Clin. Exp. Immunol. 2012, 168, 12–23. [Google Scholar] [CrossRef]
- Freund, M.W.; Kleinveld, G.; Krediet, T.G.; van Loon, A.M.; A Verboon-Maciolek, M. Prognosis for neonates with enterovirus myocarditis. Arch. Dis. Child. Fetal Neonat. Ed. 2010, 95, 206. [Google Scholar] [CrossRef]
- Arbustini, E.; Porcu, E.; Bellini, O.; Grasso, M.; Pilotto, A.; Dal, B.; Morbini, P.; Diegoli, M.; Gavazzi, A.; Specchia, G.; et al. Enteroviral infection causing fatal myocarditis and subclinical myopathy. Heart 2000, 83, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.-K.; Peter, A.K.; Xiong, D.; Narezkina, A.; Yung, A.; Dalton, N.D.; Hwang, K.-K.; Yajima, T.; Chen, J.; Knowlton, K.U. Inhibition of Coxsackievirus-associated dystrophin cleavage prevents cardiomyopathy. J. Clin. Investig. 2013, 123, 5146–5151. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Wong, B.W. Protein degradation systems in viral myocarditis leading to dilated cardiomyopathy. Cardiovasc. Res. 2009, 85, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.-W.; Austin, M.; Onodera, T.; Notkins, A.L. Virus-Induced Diabetes Mellitus. New Engl. J. Med. 1979, 300, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Wang, C.-H.; Tsai, F.-J.; Hwang, K.-P.; Chen, W.; Lin, C.-C.; Li, T.-C. Enterovirus infection is associated with an increased risk of childhood type 1 diabetes in Taiwan: A nationwide population-based cohort study. Diabetologia 2014, 58, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.H.; Honkanen, H.; Pakkanen, O.; Oikarinen, S.; Hankaniemi, M.M.; Huhtala, H.; Ruokoranta, T.; Lecouturier, V.; André, P.; Harju, R.; et al. Coxsackievirus B1 Is Associated With Induction of -Cell Autoimmunity That Portends Type 1 Diabetes. Diabetes 2013, 63, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Sioofy-Khojine, A.-B.; Lehtonen, J.; Nurminen, N.; Laitinen, O.H.; Oikarinen, S.; Huhtala, H.; Pakkanen, O.; Ruokoranta, T.; Hankaniemi, M.M.; Toppari, J.; et al. Coxsackievirus B1 infections are associated with the initiation of insulin-driven autoimmunity that progresses to type 1 diabetes. Diabetologia 2018, 61, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Lowenfels, A.B.; Morselli-Labate, A.M.; Maisonneuve, P.; Pezzilli, R. Pancreatic cancer in chronic pancreatitis; Aetiology, incidence, and early detection. Best Pr. Res. Clin. Gastroenterol. 2010, 24, 349–358. [Google Scholar] [CrossRef]
- Gern, J.E.; Busse, W.W. Association of Rhinovirus Infections with Asthma. Clin. Microbiol. Rev. 1999, 12, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Samuelson, A.; Forsgren, M.; Sällberg, M. Characterization of the recognition site and diagnostic potential of an enterovirus group-reactive monoclonal antibody. Clin. Diagn. Lab. Immunol. 1995, 2, 385–386. [Google Scholar] [CrossRef] [Green Version]
- Saarinen, N.V.V.; Stone, V.M.; Hankaniemi, M.M.; Mazur, M.A.; Vuorinen, T.; Flodström-Tullberg, M.; Hyöty, H.; Hytönen, V.P.; Laitinen, O.H. Antibody Responses against Enterovirus Proteases are Potential Markers for an Acute Infection. Viruses 2020, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Poelman, R.; Schuffenecker, I.; van Leer-Buter, C.; Josset, L.; Niesters, H.G.; Lina, B. European surveillance for enterovirus D68 during the emerging North American outbreak in 2014. J. Clin. Virol. 2015, 71, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Edlmayr, J.; Niespodziana, K.; Popow-Kraupp, T.; Krzyzanek, V.; Focke-Tejkl, M.; Blaas, D.; Grote, M.; Valenta, R. Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. Eur. Respir. J. 2010, 37, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, J.; Smith, W.-A.; Khoo, S.-K.; Bizzintino, J.; Zhang, G.; Cox, D.W.; Laing, I.A.; le Souef, P.; Thomas, W.R.; Hales, B.J. Comparison of rhinovirus antibody titers in children with asthma exacerbations and species-specific rhinovirus infection. J. Allergy Clin. Immunol. 2014, 134, 25–32.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelmann, I.; Mordacq, C.; Gosset, P.; Tillie-Leblond, I.; Dewilde, A.; Thumerelle, C.; Pouessel, G.; Deschildre, A. Rhinovirus and Asthma: Reinfection, Not Persistence. Am. J. Respir. Crit. Care Med. 2013, 188, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, O.H.; Svedin, E.; Kapell, S.; Hankaniemi, M.M.; Larsson, P.G.; Domsgen, E.; Stone, V.M.; Määttä, J.; Hyöty, H.; Hytönen, V.P.; et al. New Coxsackievirus 2Apro and 3Cpro protease antibodies for virus detection and discovery of pathogenic mechanisms. J. Virol. Methods 2018, 255, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, O.H.; Airenne, K.J.; Hytönen, V.P.; Peltomaa, E.; Mähönen, A.J.; Wirth, T.; Lind, M.M.; Mäkelä, K.A.; Toivanen, P.I.; Schenkwein, D.; et al. A multipurpose vector system for the screening of libraries in bacteria, insect and mammalian cells and expression in vivo. Nucleic Acids Res. 2005, 33, e42. [Google Scholar] [CrossRef] [Green Version]
- Oberbichler, E.; Wiesauer, M.; Schlögl, E.; Stangl, J.; Faschinger, F.; Knör, G.; Gruber, H.J.; Hytönen, V.P. Chapter One—Competitive binding assay for biotin and biotin derivatives, based on avidin and biotin-4-fluorescein. Methods Enzymol. 2020, 633, 1–20. [Google Scholar]
- Ray, S.; Steven, R.T.; Green, F.M.; Höök, F.; Taskinen, B.; Hytönen, V.P.; Shard, A.G. Neutralized Chimeric Avidin Binding at a Reference Biosensor Surface. Langmuir 2015, 31, 1921–1930. [Google Scholar] [CrossRef]
- Näntö-Salonen, K.; Kupila, A.; Simell, S.; Siljander, H.; Salonsaari, T.; Hekkala, A.; Korhonen, S.; Erkkola, R.; I Sipilä, J.; Haavisto, L.; et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: A double-blind, randomised controlled trial. Lancet 2008, 372, 1746–1755. [Google Scholar] [CrossRef]
- Grönroos, M.; Parajuli, A.; Laitinen, O.H.; Roslund, M.; Vari, H.K.; Hyöty, H.; Puhakka, R.; Sinkkonen, A. Short-term direct contact with soil and plant materials leads to an immediate increase in diversity of skin microbiota. Microbiologiopen 2018, 8, e00645. [Google Scholar] [CrossRef]
- Saarinen, N.V.V.; Laiho, J.E.; Richardson, S.J.; Zeissler, M.; Stone, V.M.; Marjomäki, V.S.; Kantoluoto, T.; Horwitz, M.S.; Sioofy-Khojine, A.-B.; Honkimaa, A.; et al. A novel rat CVB1-VP1 monoclonal antibody 3A6 detects a broad range of enteroviruses. Sci. Rep. 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Vandini, S.; Biagi, C.; Fischer, M.; Lanari, M. Impact of Rhinovirus Infections in Children. Viruses 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodbury, N.; Bald, E.; Geist, B.; Yang, T.-Y. Application of multiplexed pharmacokinetic immunoassay to quantify in vivo drug forms and coadministered biologics. Bioanalysis 2019, 11, 2251–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meso Scale Diagnostics. Available online: https://www.mesoscale.com/en/products_and_services/assay_kits/u-plex_gateway/u-plex_technology (accessed on 6 August 2019).
- Stone, V.M.; Hankaniemi, M.M.; Laitinen, O.H.; Sioofy-Khojine, A.B.; Lin, A.; Lozano, I.M.D.; Mazur, M.A.; Marjomäki, V.; Loré, K.; Hyöty, H.; et al. A hexavalent Coxsackievirus B vaccine is highly immunogenic and has a strong protective capacity in mice and nonhuman primates. Sci. Adv. 2020, 6, eaaz2433. [Google Scholar] [CrossRef] [PubMed]
- Hyöty, H.; Leon, F.; Knip, M. Developing a vaccine for type 1 diabetes by targeting coxsackievirus B. Expert Rev. Vaccines 2018, 17, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Niespodziana, K.; Napora, K.; Cabauatan, C.; Focke-Tejkl, M.; Keller, W.; Niederberger, V.; Tsolia, M.; Christodoulou, I.; Papadopoulos, N.G.; Valenta, R. Misdirected antibody responses against an N-terminal epitope on human rhinovirus VP1 as explanation for recurrent RV infections. FASEB J. 2011, 26, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Francis, T. On the Doctrine of Original Antigenic Sin. Proc. Am. Philos. Soc. 1960, 104, 572–578. [Google Scholar]
- Zhang, A.; Stacey, H.D.; Mullarkey, C.E.; Miller, M.S. Original Antigenic Sin: How First Exposure Shapes Lifelong Anti–Influenza Virus Immune Responses. J. Immunol. 2019, 202, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Pattison, J.R. Editorial: Tests for Coxsackie B Virus-Specific IgM. J. Hyg. 1983, 90, 327–332. [Google Scholar] [CrossRef] [Green Version]





| Sample Group | n |
|---|---|
| Adult IgM pos 1 | 20 |
| Adult IgM neg 1 | 22 |
| Adult biobank | 20 |
| Children 2 | 88 |
| Antigen | p | p Adj.1 |
|---|---|---|
| CVA4 | 0.113 | N.S. |
| CVB1 | 0.167 | N.S. |
| PV1 | 0.082 | N.S. |
| EV-D68 | 0.048 | 0.477 |
| RV-A89 | 0.252 | N.S. |
| RV-B14 | 0.167 | N.S. |
| RV-C3 | 0.116 | N.S. |
| 2A | 0.003 | 0.032 |
| 3C | 0.030 | 0.296 |
| BSA | 0.470 | N.S. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saarinen, N.V.V.; Lehtonen, J.; Veijola, R.; Lempainen, J.; Knip, M.; Hyöty, H.; Laitinen, O.H.; Hytönen, V.P. Multiplexed High-Throughput Serological Assay for Human Enteroviruses. Microorganisms 2020, 8, 963. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8060963
Saarinen NVV, Lehtonen J, Veijola R, Lempainen J, Knip M, Hyöty H, Laitinen OH, Hytönen VP. Multiplexed High-Throughput Serological Assay for Human Enteroviruses. Microorganisms. 2020; 8(6):963. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8060963
Chicago/Turabian StyleSaarinen, Niila V. V., Jussi Lehtonen, Riitta Veijola, Johanna Lempainen, Mikael Knip, Heikki Hyöty, Olli H. Laitinen, and Vesa P. Hytönen. 2020. "Multiplexed High-Throughput Serological Assay for Human Enteroviruses" Microorganisms 8, no. 6: 963. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8060963