1. Introduction
Okara is a food processing by-product derived from soybeans (
Glycine max), being the solid residue that remains after filtering the water-soluble fraction during soymilk or soybean curd (tofu) production [
1]. About 1.2 kg of okara is obtained from every 1 kg of soybeans that are used in the tofu production [
2]. Therefore, large amounts of okara are produced annually, especially in Asian countries with high soybean consumption. The amount of okara produced by the tofu manufacturing sector is about 800,000 t in Japan; 310,000 t in Korea; and 280,0000 t in China. Even with the large volumes of okara generated by the food industry, most of it is discarded due to the okara’s high moisture content, which makes it very perishable [
3,
4]. This by-product is a rich source of high-quality proteins, carbohydrates, unsaturated lipids, and dietary fiber, and also contains isoflavones, minerals, and oligosaccharides [
4]. Therefore, okara is a potential inexpensive functional food ingredient [
5]. However, its rate of reuse is still low and is intended almost exclusively for animal feed [
2,
6] or human food, with some limitations due to its oligosaccharide content, which produces flatulence and the disagreeable ‘fishy’ and ‘beany’ flavor, whereas the rest is discarded as industrial garbage [
1].
Annually, in Europe, countries like Germany, France, the UK, and Spain are processing approximately 16 million tons of animal fat by-products [
7]. The pork lard was associated for a long time as a potential cause of vascular and heart disease [
8] and therefore not used as much in food preparation. The price of animal fat is relatively low, which makes it a cheap and promising substrate for microbial fermentation [
9] in order to obtain valuable metabolites (i.e., short- and medium-chain fatty acids) [
10].
Solid-state fermentation (SSF) is a conventional fermentation method that may be designed to improve the nutritional value and functional properties of food processing by-products [
1]. The biological activities of okara can be improved by SSF due to the fact that the microbial proteases are able to metabolize the high molecular weight proteins to smaller, bioactive peptides or amino acids. Additionally, the microbial lipase can metabolize the pork fat in order to generate the valuable fatty acids. Okara was used as a fermentation substrate to produce a variety of products for human consumption including α-glucosidase inhibitors that are used in the diabetes mellitus treatment, β-fructofuranosidase, fibrinolytic enzymes, iturin A, edible fungi, chitosan, alcohol, and biosurfactants [
4]. Additionally, okara can support probiotic growth and survival in different formulated media and under simulated gastrointestinal conditions [
5]. Additionally, Vong and Liu (2016) [
3] reported a study that regards the fermented okara with improved nutritional value, its aroma compounds, and its high antioxidant potential.
Yarrowia lipolytica is an excellent microorganism with multiple biotechnological applications [
11]. On the other hand, lactic acid bacteria (LAB), belonging to the
Lactobacillus spp. genus., are considered important in relation to the biodegradation of fatty materials, thus enhancing the digestibility of proteins and supporting human health by inhabiting the gastrointestinal tract. These properties of LAB are attributed to their metabolic processes including enzymes such as lipases, proteases, and antibacterial proteins [
12]. Both
Y. lipolytica and
Lactobacillus spp. are used in the production of fermented products due to their generally recognized as safe (GRAS) status [
6,
13]. Fermentation using microorganisms in a co-culture such as LAB and yeast could provide promising results such as an increase in the microbial safety status of the products by reducing the potential spore forming microorganism [
4] due to the production of organic acids and other inhibitory substances [
3]. Thus, due to their strong metabolic activity and diversity, the co-fermentation of yeast and lactobacilli offers great potential for the biotransformation of okara and pork lard. However, to the best of our knowledge, the fermentation of both okara and pork lard in a yeast and lactobacilli co-culture has not been investigated previously. Instead, Tzirita et al. (2018) [
10] reported on waste fat biodegradation by
Y. lipolytica,
Bacillus spp. and
Pseudomonas putida consortium in order to produce lipids with significant industrial, ecological, and biotechnological interest.
The aim of this study was to investigate the microbial transformation of the freeze-dried okara and pork lard as a valuable biotechnological way for the economically attractive production of value-added, functional products for food and feed applications by using a co-culture of Y. lipolytica and L. paracasei. First, the selection of the yeast strains based on their capacity to biosynthesize lipases and proteases was performed. Second, different microbial consortia for solid state fermentation of the substrates were tested. Furthermore, the identification of the parameters that influence the SSF fermentation in order to obtain a fermented product with improved antioxidant and antimicrobial potential was achieved by applying the Plackett–Burman experimental design.
2. Materials and Methods
2.1. Materials and Microorganisms
Soybeans not genetically modified were purchased from Solaris Plant S.R.L., Bucharest, Romania. The okara was obtained through the method described by Vong and Liu (2016) [
3] with some modifications. The grains were hydrated with tap water in a ratio of 1:6 (
w/
v), for 16 h at 4 °C, followed by grinding for 10 min and filtering. The solid by-product was lyophilized using the Alpha 1–4 equipment (Martin Christ, Osterode am Harz, Germany), followed by grinding. The resulting powder contained 27.3% carbohydrates, 26.4% proteins, 21.7% dietary fiber, 18.3% lipids, and 4.3% ash. The pork lard was procured from a local artisanal farm.
Nineteen yeast strains of Y. lipolytica, Candida lipolytica, C. krusei, and C. colliculosa and one strain of L. paracasei were used. These strains are part of the Collection of Microorganisms (acronym MIUG) of Bioaliment Research Platform from the Faculty of Food Science and Engineering, “Dunărea de Jos” University, Galați, Romania. One of the yeast strains, Y. lipolytica ATCC 18942, was purchased from the American Type Culture Collection (ATCC), Manassas, VA, USA.
To test the antimicrobial activity, Aspergillus niger MIUG M5 and Bacillus subtilis MIUG B1 strains were used as indicator microorganisms. All the chemicals, reagents, and commercial culture media were purchased from Sigma-Aldrich (Steinheim, Germany).
Bottom of Form.
2.2. Selection of the Yeast Strains as Lipase and Protease Producers
The Y. lipolytica, C. lipolytica, C. krusei, and C. colliculosa yeast strains were studied for their capacity to produce lipases and proteases. To underline the lipase activity, the following selective media was used (w/v%): pork lard 5.0, peptone 0.5, yeast extract 0.25, agar 2.0, and pH 6.5. Sequentially, to detect the proteolytic strains, the following medium was used (w/v%): freeze-dried okara 2.0 and agar 2.0, pH 6.5.
To assess the hydrolases (protease and lipase) activity assay, the radial diffusion method was used.
Therefore, for the lipase activity, the specific media was supplemented with 1% Rhodamine B (1 mg/mL) and inoculated with the yeast cells [
14]. After incubation at 28 °C for five days, an orange fluorescent halo visible upon UV irradiation (Ĉ = 350 nm) around the active yeast colonies appeared [
15]. The colony diameter was measured and expressed in mm.
Furthermore, the lipolytic activity of the most active strains of
Y. lipolytica was assayed by the plate diffusion method, in the spirit blue agar medium (Difco), according to the method of Parfene et al. (2012) [
16] with some modifications. The rehydrated commercial media in accordance with the manufacturer was supplemented with 2% pork lard. The pH of the media was adjusted to 5.0, sterilized at 121 °C for 15 min, and was then centrally inoculated with the yeast biomass. The growth was performed at 28 °C for 48 h. The lipolysis was assessed by observing the halos on the plate, which in turn indicated that the selected microorganisms metabolized the lipids [
17]. The hydrolysis index (HI) was determined as the ratio between the diameter of the hydrolysis zone and the diameter of the colony.
Regarding the protease activity, after the incubation at 28 °C for five days, the most active strains were highlighted through the appearance of a clear zone around the colonies, compared to the rest of the medium that was opaque [
18]. The HI was also taken into account.
2.3. Inoculum Preparation and Solid State Fermentation (SSF) Fermentation
All the stock cultures were preserved in 40% glycerol at −80 °C. The yeast strains were reactivated on a yeast extract peptone dextrose (YPD) agar medium for 3–5 days, at 28 °C. In order to obtain the inoculum, the yeast biomass was transferred into 50 mL sterile physiological solution (0.9% NaCl). Then, the yeast cells were counted with the Thoma cytometer and inoculated in the fermentation medium at a final concentration of 1.0 × 107 CFU/g.
The LAB culture was reactivated on De Man, Rogosa and Sharpe broth (MRS) for 48 h at 37 °C. A 2% inoculum with an optical density (OD) of 2.601 measured at the wavelength of 600 nm with a UV/VIS V-530 spectrophotometer (Jasco, Tokyo, Japan).
2.3.1. SSF Fermentation with the Monoculture of Y. lipolytica
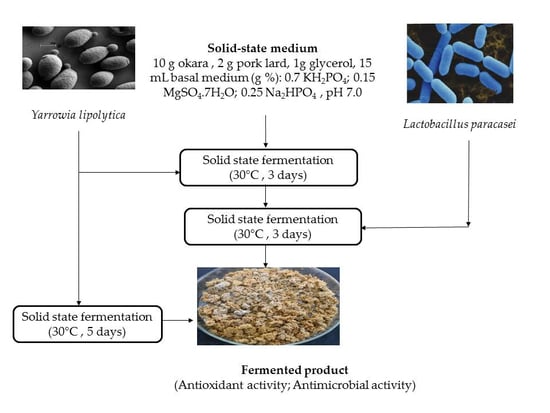
The cultivation in the SSF system was prepared in sterile glass Petri dishes with a diameter of 120 mm. Thus, 10.0 g of freeze-dried okara, 2.0 g of pork lard, 1.0 g of glycerol, and 15 mL of basal medium consisting of 0.7 KH2PO4, 0.25 Na2HPO4, 0.15 MgSO4 × 7H2O (w/v%), and pH 7.0 were sterilized at 121 °C for 15 min. After cooling, the medium was inoculated with 1 mL of yeast inoculum to obtain a final concentration of 1.0 × 107 CFU/g. The fermentation took place in a stationary system at 30 °C for five days. Two fermented products were developed, one with Y. lipolytica MIUG D5 and one with Y. lipolytica ATCC 18942.
2.3.2. SSF Fermentation with the Co-Culture of Y. lipolytica and L. paracasei
The yeast cultivation in a SSF system was prepared as described above with the mention that the yeast was cultivated for 72 h at 30 °C. Then, 2% of the L. paracasei MIUG BL20 inoculum was added and incubated further for another 72 h. Two fermented products were developed, one with the co-culture of Y. lipolytica MIUG D5 and L. paracasei MIUG BL20 and the other one with Y. lipolytica ATCC 18942 and L. paracasei MIUG BL20.
At the end of the fermentation, the fermented medium was subjected to freeze-drying and then tested to assess the antioxidant and antimicrobial activity.
2.4. Identifying the Significant Variables of the Biotechnological Process by Using the Plackett–Burman Design
The Plackett–Burman experimental design was used as an effective tool for the screening of the fermentation parameters with respect to their main effects on the process. The variables chosen for the present study were the freeze-dried okara, pork lard, glycerol concentration, yeast, and LAB inoculum concentration, and the fermentation time. As responses, the antioxidant and antimicrobial activities of the fermented products were considered. The experimental design for the screening of the variables is shown in
Table 1. All the variables were considered as numerical factors and investigated at two widely spaced intervals designated as −1 (low level) and +1 (high level) [
19].
2.5. Antimicrobial Activity
The antimicrobial activity [
20,
21] was assessed against two spoilage microorganisms such as
A. niger MIUG M5 and
B. subtilis MIUG B1. Each 0.5 g of the freeze-dried fermented medium was homogenized with 45 mL of sterile Potato Dextrose Agar (PDA) or Plate Count Agar (PCA) medium, cooled to 42 °C, and poured into Petri dishes. The plates were centrally inoculated with 5 µL spore suspension with a final concentration of 1 × 10
6 spores/mL and incubated at 25 °C for five days (for molds) and at 37 °C for 48 h (for bacteria). The control plates containing PDA and PCA media mixed with sterile distilled water in the same proportions as above were also prepared and inoculated. After the incubation period, the growth area in both treated (A
T) and control (A
C) plates was determined from the mean perpendicular diameter measurements by assuming a circular growth. The inhibition ratio (IR) was calculated using the following Equation (1):
2.6. Antioxidant Capacity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity for the freeze-dried fermented medium was performed [
22]. The absorbance was read at 515 nm in a UV–Vis spectrophotometer (model Libra S22, Biochrom, Cambridge, UK) [
6]. The antioxidant activity was expressed as mM Trolox equivalents (TE)/g using the Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) calibration curve as the standard.
Previously, 1 g of the freeze-dried fermented medium was mixed with 9 mL distilled water and the extraction was performed during 30 min on an ultrasonic cleaner (MRC Ltd. Laboratory Equipment, Holon, Israel). Afterward, the samples were centrifuged at 8000 rpm for 15 min at 4 °C. The supernatant was used for further analysis.
2.7. Statistical Analysis
All the experiments were performed in triplicate. The results were expressed as average values with standard deviation. The statistical analysis of the data was performed using Minitab 17 (Minitab LLC, State College, PA, USA). To assess the differences between the samples and the control, the Kruskal–Wallis nonparametric test was used. The differences between samples were determined by applying the Tukey test with the one-way analysis of variance (ANOVA) method. The analysis of variance (ANOVA) for the Plackett–Burman design of the experiments was conducted using the Design Expert software version 8.0.2.0 (Stat-Ease Inc., Minneapolis, MN, USA).
4. Discussion
The aim of this study was to valorize some agri-food processing by-products such as okara [
1] and pork lard [
9] by applying an inexpensive and conventional biotechnological method [
1], namely solid state fermentation (SSF) using a co-culture of
Y. lipolytica and
L. paracasei in order to obtain several functional fermented products that have been proposed for use in the food and feed industry [
2,
6]. These fermentative substrates were chosen because of the annually tons of okara that are generated, especially in Asian countries, and tons of pork lard that are generated in industrialized countries from Europe.
The solid-state fermentation using the GRAS recognized
Y. lipolytica and
L. paracasei [
6,
13], the nutritional and functional values of the okara and pork lard [
4,
10], may be significantly improved [
1] by generating bioactive peptides and organic acids including fatty acids with antimicrobial potential [
3]. To the best of our knowledge, no information has been found in the literature about the conversion of both okara and pork lard through SSF fermentation using a co-culture of
Y. lipolytica and
L. paracasei. Therefore, in our study, first, the okara and pork lard were subjected to proteolysis and lipolysis by the extracellular enzymes synthesized by the selected yeast strains, in order to obtain small molecules [
23], and afterward, the
L. paracasei strain could easily metabolize this type of molecules. In order to identify the potential yeast strains that are able to produce lipase and protease, the selection of the strains on specific substrates (5% pork lard and 2% freeze-dried okara) was accomplished.
Our results are in good agreement with the data that reported that the non-conventional
Y. lipolytica yeast was able to produce extracellular lipases and proteases [
17,
24]. The first lipase obtained by converting pork lard for biorefinery was reported by Lopes et al. (2018) [
9]. Additionally, Szotkowski et al. (2019) [
7] studied four red yeast (
Cystofilobasidium macerans CCY 10-1-2,
Rhodotorula glutinis CCY 20-2-26,
R. mucilaginosa CCY 19-4-6, and
Sporobolomyces pararoseus CCY 19-9-6) that were able to produce an extracellular lipase by using animal fat as a substrate. Moreover, another study also described other red yeast strains (
Rhodotorula spp.,
Sporobolomyces spp.,
Cystofilobasidium spp.) that are able to produce polyunsaturated fatty acids from animal fat waste [
25].
From our previous results, several yeast strains were identified as active producers of lipase and protease by using bovine colostrum and cow milk fat as substrates [
26,
27,
28].
Y. lipolytica A-101 and W29 produced a lipase after 54 h of incubation at 25 °C (2.3 mm) on the spirit blue agar medium supplemented with butter fat [
29].
The antimicrobial activity of the fermented products could be correlated with the organic acids produced by the LAB and yeast strains that are responsible for the growth inhibition of the aerobe spore forming microorganisms. Hence, the antimicrobial potential of the fermented products could be associated with the production of organic acids, also including small chain saturated fatty acids, peptides (bacteriocins), carbon dioxide, hydrogen peroxide, ethanol, and diacetyl [
30]. Our previous results stated that the fermented product obtained by bovine colostrum fermentation with kefir grains enhanced with a selected
Candida lypolitica inoculum showed an inhibition zone ranging between 3.0 and 5.0 mm against
B. subtilis MIUG B1, while for
A. niger MIUG M64, spore inhibition was observed [
26,
28].
The DPPH radical scavenging activity of the fermented product derived from the principal substrates, okara and pork lard, increased during fermentation. Our results were in accordance with Patrignani et al. (2011) [
31], who described that the fermented okara showed the highest DPPH radical scavenging activity of 43.68 µg TE/g dried okara after 72 h of fermentation with
B. subtilis WX-17. Compared to the DPPH radical scavenging activity of the unfermented okara of 6.79 µg TE/g dried okara, the fermented okara displayed an increase of the DPPH radical scavenging activity by approximately 6.4 times.
Taking into account the obtained results and the novelty of this approach, the selection of the most important parameters that influence the SSF fermentation of agri-food by-product substrates using a co-culture of Y. lipolytica MIUG D5 and L. paracasei MIUG BL20 was performed. The statistical analysis highlighted that the concentration of the pork lard influenced the antioxidant activity of the fermented product, while the antimicrobial activity was determined by the time of fermentation with the selected yeasts.
The effects of temperature, water activity, and yeast inoculation level upon the lipolytic pattern and volatile production by
Y. lipolytica Y16A, according to a central composite design (CCD) using pork fat as a substrate, was assessed by Patrignani et al. (2011) [
31]. Another study underlined the effect of pH, pork lard concentration, arabic gum concentration, and oxygen mass transfer rate on the metabolic activity of the
Y. lipolytica strain [
9].
Based on the Plackett–Burman design and analysis, the most important biotechnological parameters that influence the metabolic activities of the yeasts and lactobacili cultivated in a co-culture in order to obtain a fermented product with improved antioxidant and antimicrobial activities are the time of the yeast fermentation and the concentration of pork lard.
Further experiments will take into account the optimization of the most important factors that influence the obtainment of the functional fermented product by using the CCD and the response surface methodology (RSM) tools. Then, the identification and characterization of the compounds responsible for the antimicrobial and antioxidant activity will be taken into consideration as well as organic acids including the short chain saturated fatty acids as well as some valuable bioactive peptides. The fermented product is recommended for use as a food or feed ingredient with biotic properties in order to improve the functional composition, the shelf life, and the safety assurance. From an economic point of view, the proposed biotechnological process is cheap and quite feasible for the valorization of agri-food by-products. Thus, in accordance with the principles of a circular economy, the valorization of waste into new functional bioproducts is a modern emergent task that offers new approachable applications.