Toxoplasma gondii Genotypes Circulating in Serbia—Insight into the Population Structure and Diversity of the Species in Southeastern Europe, a Region of Intercontinental Strain Exchange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. T. gondii gDNA Detection
2.3. Bioassay in Swiss Webster Mice for Strain Isolation
2.4. Nucleic Acids Extraction
2.5. DNA Precipitation by Na-Acetate/Ethanol
2.6. Genotyping by MnPCR-RFLP and Genotype Identification
2.7. Phylogenetic Analysis
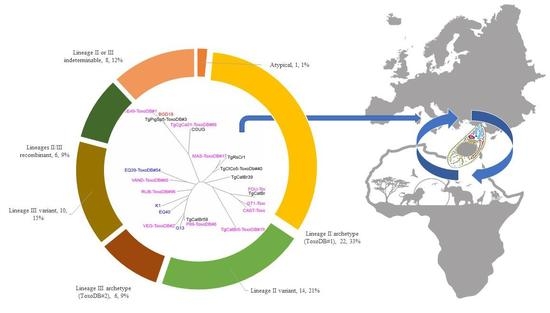
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites; Microbiological Risk Assessment Series; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2014; Volume 23. [Google Scholar]
- Bouwknegt, M.; Devleesschauwer, B.; Graham, H.; Robertson, L.J.; van der Giessen, J.W. The Euro-FBP Workshop participants. Prioritisation of food-borne parasites in Europe, 2016. Eurosurveillance 2018, 9, 17-00161. [Google Scholar] [CrossRef] [Green Version]
- Dubey, J.P.; Lindsay, D.S.; Speer, C.A. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 1998, 11, 267–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, L.M.; Dubey, J.P. Toxoplasmosis: A history of clinical observations. Int. J. Parasitol. 2009, 39, 895–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowie, W.R.; King, A.S.; Werker, D.H.; Isaac-Renton, J.L.; Bell, A.; Eng, S.B.; Marion, S.A. Outbreak of toxoplasmosis associated with municipal drinking water. The BC Toxoplasma Investigation Team. Lancet 1997, 350, 173–177. [Google Scholar] [CrossRef]
- Benenson, M.W.; Takafuji, E.T.; Lemon, S.M.; Greenup, R.L.; Sulzer, A.J. Oocyst-transmitted toxoplasmosis associated with ingestion of contaminated water. N. Engl. J. Med. 1982, 307, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bahia-Oliveira, L.M.; Jones, J.L.; Azevedo-Silva, J.; Alves, C.C.; Orefice, F.; Addiss, D.G. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg. Infect. Dis. 2003, 9, 55–62. [Google Scholar] [CrossRef]
- Frenkel, J.K.; Dubey, J.P.; Miller, N.L. Toxoplasma gondii in cats: Fecal stages identified as coccidian oocysts. Science 1970, 6, 893–896. [Google Scholar] [CrossRef]
- Dubey, J.P.; Miller, N.L.; Frenkel, J.K. Toxoplasma gondii life cycle in cats. J. Am. Vet. Med. Assoc. 1970, 157, 1767–1770. [Google Scholar]
- Sepúlveda-Arias, J.C.; Gómez-Marin, J.E.; Bobić, B.; Naranjo-Galvis, C.A.; Djurković-Djaković, O. Toxoplasmosis as a travel risk. Travel Med. Infect. Dis. 2014, 6 Pt A, 592–601. [Google Scholar] [CrossRef]
- Henao-Martínez, A.F.; Franco-Paredes, C.; Palestine, A.G.; Montoya, J.G. Symptomatic Acute Toxoplasmosis in Returning Travelers. Open Forum Infect. Dis. 2018, 5, ofy058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwab, E.; Zhu, X.; Majumdar, D.; Pena, H.; Gennari, S.; Dubey, J.; Su, C. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 2014, 141, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Shwab, E.K.; Saraf, P.; Zhu, X.Q.; Zhou, D.H.; McFerrin, B.M.; Ajzenberg, D.; Schares, G.; Hammond-Aryee, K.; van Helden, P.; Higgins, S.A.; et al. Human impact on the diversity and virulence of the ubiquitous zoonotic parasite Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2018, 115, E6956–E6963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Dubey, J.P.; Su, C.; Ajioka, J.W.; Rosenthal, B.M.; Sibley, L.D. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int. J. Parasitol. 2011, 41, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Pena, H.F.; Gennari, S.M.; Dubey, J.P.; Su, C. Population structure and mouse-virulence of Toxoplasma gondii in Brazil. Int. J. Parasitol. 2008, 38, 561–569. [Google Scholar] [CrossRef]
- Mercier, A.; Devillard, S.; Ngoubangoye, B.; Bonnabau, H.; Bañuls, A.L.; Durand, P.; Salle, B.; Ajzenberg, D.; Dardé, M.L. Additional haplogroups of Toxoplasma gondii out of Africa: Population structure and mouse-virulence of strains from Gabon. PLoS Negl. Trop. Dis. 2010, 4, e876. [Google Scholar] [CrossRef] [Green Version]
- Hamidović, A.; Etougbétché, J.R.; Tonouhewa, A.B.N.; Galal, L.; Dobigny, G.; Houémènou, G.; Da Zoclanclounon, H.; Amagbégnon, R.; Laleye, A.; Fievet, N.; et al. A hotspot of Toxoplasma gondii Africa 1 lineage in Benin: How new genotypes from West Africa contribute to understand the parasite genetic diversity worldwide. PLoS Negl. Trop. Dis. 2021, 15, e0008980. [Google Scholar] [CrossRef] [PubMed]
- Galal, L.; Hamidović, A.; Dardé, M.L.; Mercier, M. Diversity of Toxoplasma gondii strains at the global level and its determinants. Food Waterborne Parasitol. 2019, 15, e00052. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shwab, E.K.; Martin, R.M.; Gerhold, R.W.; Rosenthal, B.M.; Dubey, J.P.; Su, C. A partition of Toxoplasma gondii genotypes across spatial gradients and among host species, and decreased parasite diversity towards areas of human settlement in North America. Int. J. Parasitol. 2018, 48, 611–619. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Liu, D.; Huo, X.; Gao, J.; Song, X.; Xu, X.; Huang, K.; Liu, W.; Wang, Y.; et al. Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS ONE 2013, 8, e53483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lélu, M.; Villena, I.; Dardé, M.L.; Aubert, D.; Geers, R.; Dupuis, E.; Marnef, F.; Poulle, M.L.; Gotteland, C.; Dumètre, A.; et al. Quantitative estimation of the viability of Toxoplasma gondii oocysts in soil. Appl. Environ. Microbiol. 2012, 78, 5127–5132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uzelac, A.; Klun, I.; Cirkovic, V.; Djurkovic-Djakovic, O. In Vivo and In Vitro Virulence Analysis of Four Genetically Distinct Toxoplasma gondii Lineage III Isolates. Microorganisms 2020, 8, 1702. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.; Ivović, V.; Štajner, T.; Djokić, V.; Klun, I.; Bobić, B.; Nikolić, A.; Djurković-Djaković, O. Evidence for genetic diversity of Toxoplasma gondii in selected intermediate hosts in Serbia. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 173–179. [Google Scholar] [CrossRef]
- Djurković-Djaković, O.; Nikolić, A.; Bobić, B.; Klun, I.; Aleksić, A. Stage conversion of Toxoplasma gondii RH parasites in mice by treatment with atovaquone and pyrrolidine dithiocarbamate. Microbes Infect. 2005, 7, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Klun, I.; Uzelac, A.; Villena, I.; Mercier, A.; Bobić, B.; Nikolić, A.; Rajnpreht, I.; Opsteegh, M.; Aubert, D.; Blaga, R.; et al. The first isolation and molecular characterization of Toxoplasma gondii from horses in Serbia. Parasit. Vectors 2017, 10, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.; Shwab, E.K.; Zhou, P.; Zhu, X.Q.; Dubey, J.P. Moving towards and integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 2010, 137, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Taylor, S.; Su, C.; Mackey, A.J.; Boyle, J.; Cole, R.; Glover, D.; Tang, K.; Paulsen, I.T.; Berriman, M.; et al. Composite genome map and recombination parameters derived from three archetypal lineages of Toxoplasma gondii. Nucleic Acids Res. 2005, 33, 2980–2992. [Google Scholar] [CrossRef] [Green Version]
- Howe, D.K.; Sibley, L.D. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J. Infect. Dis. 1995, 172, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, X.; Dubey, J.P. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: A high resolution and simple method for identification of parasites. Int. J. Parasitol. 2006, 36, 841–848. [Google Scholar] [CrossRef]
- Genot, S.; Franck, J.; Forel, J.M.; Rebaudet, S.; Ajzenberg, D.; de Paula, A.M.; Dardé, M.L.; Stein, A.; Ranque, S. Severe Toxoplasma gondii I/III recombinant-genotype encephalitis in a human immunodeficiency virus patient. J. Clin. Microbiol. 2007, 45, 3138–3140. [Google Scholar] [CrossRef] [Green Version]
- Castro, B.B.P.; Gennari, S.M.; Lorenzi, H.; Su, C. A simple method to generate PCR-RFLP typing profiles from DNA sequences in Toxoplasma gondii. Infect. Genet. Evol. 2020, 85, 104590. [Google Scholar] [CrossRef]
- Fernández-Escobar, M.; Calero-Bernal, R.; Regidor-Cerrillo, J.; Vallejo, R.; Benavides, J.; Collantes-Fernández, E.; Ortega-Mora, L.M. Isolation, Genotyping, and Mouse Virulence Characterization of Toxoplasma gondii From Free Ranging Iberian Pigs. Front. Vet. Sci. 2020, 7, 604782. [Google Scholar] [CrossRef]
- Štajner, T.; Vasiljević, Z.; Vujić, D.; Marković, M.; Ristić, G.; Mićić, D.; Pasić, S.; Ivović, V.; Ajzenberg, D.; Djurković-Djaković, O. Atypical strain of Toxoplasma gondii causing fatal reactivation after hematopoietic stem cell transplantion in a patient with an underlying immunological deficiency. J. Clin. Microbiol. 2013, 51, 2686–2690. [Google Scholar] [CrossRef] [Green Version]
- Darde, M.-L.; Passebosc, K.; Durieux, M.-F.; Boumediene, F.; Galal, L.; Mercier, A.; The Network of the French National Reference Center for Toxoplasmosis. Analysing the genetic diversity of Toxoplasma gondii in an European country via human samples. In Programme & Abstract Book of the 13th European Multicolloquium of Parasitology, Belgrade, Serbia, 12–16 October 2021; Klun, I., Djurković-Djaković, O., Eds.; Serbian Society for Parasitology: Belgrade, Serbia, 2021; p. 88. [Google Scholar]
- Caner, A.; Ajzenberg, D.; Değirmenci, A.; Darde, M.L.; Can, H.; Erdoğan, D.D.; Korkmaz, M.; Uner, A.; Güngör, C.; Altıntaş, K.; et al. Isolation of Toxoplasma gondii strains similar to Africa 1 genotype in Turkey. Parasitol. Int. 2013, 62, 471–474. [Google Scholar] [CrossRef]
- Can, H.; Doskaya, M.; Ajzenberg, D.; Ozdemir, H.G.; Caner, A.; Iz, S.G.; Doskaya, A.D.; Atalay, E.; Cetinkaya, C.; Urgen, S.; et al. Genetic characterization of Toxoplasma gondii isolates and toxoplasmosis seroprevalence in stray cats of İzmir, Turkey. PLoS ONE 2014, 9, e104930. [Google Scholar] [CrossRef] [Green Version]
- Burrells, A.; Bartley, P.M.; Zimmer, I.A.; Roy, S.; Kitchener, A.C.; Meredith, A.; Wright, S.E.; Innes, E.A.; Katzer, F. Evidence of the three main clonal Toxoplasma gondii lineages from wild mammalian carnivores in the UK. Parasitology 2013, 140, 1768–1776. [Google Scholar] [CrossRef]
- Turčeková, L.; Hurníková, Z.; Spišák, F.; Miterpáková, M.; Chovancová, B. Toxoplasma gondii in protected wildlife in the Tatra National Park (TANAP), Slovakia. Ann. Agric. Environ. Med. 2014, 21, 235–238. [Google Scholar] [CrossRef]
- Ajzenberg, D.; Yera, H.; Marty, P.; Paris, L.; Dalle, F.; Menotti, J.; Aubert, D.; Franck, J.; Bessières, M.H.; Quinio, D.; et al. Genotype of 88 Toxoplasma gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. J. Infect. Dis. 2009, 199, 1155–1167. [Google Scholar] [CrossRef] [Green Version]
- Pomares, C.; Devillard, S.; Holmes, T.H.; Olariu, T.R.; Press, C.J.; Ramirez, R.; Talucod, J.; Estran, R.; Su, C.; Dubey, J.P.; et al. Genetic Characterization of Toxoplasma gondii DNA Samples Isolated from Humans Living in North America: An Unexpected High Prevalence of Atypical Genotypes. J. Infect. Dis. 2018, 218, 1783–1791. [Google Scholar] [CrossRef]
- Paunovic, M.; Cirovic, D.; Milenkovic, M. Status, management and conservation of large carnivores in Serbia. In Conference paper of Coexistence of large carnivores and humans: Threat or benefit? In Proceedings of the International Symposium Preceding the 54th CIC General Assembly, Belgrade, Serbia, 1 May 2007; pp. 111–117. [Google Scholar]
- Penezic, A.; Selakovic, S.; Pavlovic, I.; Cirovic, D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014, 113, 3281–3285. [Google Scholar] [CrossRef]
- Lozano, J.; Malo, A.F. Conservation of the European wildcat (Felis silvestris) in Mediterranean environments: A reassessment of current threats. In Mediterranean Ecosystems: Dynamics, Management and Conservation; Williams, G.S., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 1–31. [Google Scholar]
- Uzelac, A.; Klun, I.; Cirovic, D.; Penezic, A.; Cirkovic, V.; Djurkovic-Djakovic, O. Detection and genotyping of Toxoplasma gondii in wild canids in Serbia. Parasitol. Int. 2019, 73, 101973. [Google Scholar] [CrossRef]
- Sgroi, G.; Viscardi, M.; Santoro, M.; Borriello, G.; D’Alessio, N.; Boccia, F.; Pacifico, L.; Fioretti, A.; Veneziano, V.; Fusco, G. Genotyping of Toxoplasma gondii in wild boar (Sus scrofa) in southern Italy: Epidemiological survey and associated risk for consumers. Zoonoses Public Health 2020, 67, 805–813. [Google Scholar] [CrossRef]
- Schumacher, A.C.; Elbadawi, L.I.; DeSalvo, T.; Straily, A.; Ajzenberg, D.; Letzer, D.; Moldenhauer, E.; Handly, T.L.; Hill, D.; Dardé, M.L.; et al. Toxoplasmosis Outbreak Associated with Toxoplasma gondii-Contaminated Venison—High Attack Rate, Unusual Clinical Presentation, and Atypical Genotype. Clin. Infect. Dis. 2021, 72, 1557–1565. [Google Scholar] [CrossRef]
- Demar, M.; Ajzenberg, D.; Maubon, D.; Djossou, F.; Panchoe, D.; Punwasi, W.; Valery, N.; Peneau, C.; Daigre, J.L.; Aznar, C.; et al. Fatal outbreak of human toxoplasmosis along the Maroni River: Epidemiological, clinical, and parasitological aspects. Clin. Infect. Dis. 2007, 45, e88–e95. [Google Scholar] [CrossRef] [Green Version]
- Carme, B.; Demar, M.; Ajzenberg, D.; Dardé, M.L. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg. Infect. Dis. 2009, 15, 656–658. [Google Scholar] [CrossRef]
- De Sousa, S.; Ajzenberg, D.; Canada, N.; Freire, L.; da Costa, J.M.C.; Dardé, M.L.; Thulliez, P.; Dubey, J.P. Biologic and molecular characterization of Toxoplasma gondii isolates from pigs from Portugal. Vet. Parasitol. 2005, 135, 133–136. [Google Scholar] [CrossRef]
- Halos, L.; Thébault, A.; Aubert, D.; Thomas, M.; Perreta, C.; Geers, R.; Alliota, A.; Escotte-Binet, S.; Ajzenberg, D.; Dardé, M.L.; et al. An innovative survey underlining the significant level of contamination by Toxoplasma gondii of ovine meat consumed in France. Int. J. Parasitol. 2010, 40, 193–200. [Google Scholar] [CrossRef]
- Dubey, J.P.; VanWhy, K.; Verma, S.K.; Choudhary, S.; Kwok, O.C.; Khan, A.; Behnke, M.S.; Sibley, L.D.; Ferreira, L.R.; Oliveira, S.; et al. Genotyping Toxoplasma gondii from wildlife in Pennsylvania and identification of natural recombinants virulent to mice. Vet. Parasitol. 2014, 200, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, T.; Marcet, P.L.; Graham, D.H.; Dahl, E.R.; Dubey, J.P. Globalization and the population structure of Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 2006, 103, 11423–11428. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, G.V.; Dubey, J.P.; Su, C. Genotyping studies of Toxoplasma gondii isolates from Africa revealed that the archetypal clonal lineages predominate as in North America and Europe. Vet. Parasitol. 2008, 155, 314–318. [Google Scholar] [CrossRef]
- Hahn, S.; Bauer, S.; Liechti, F. The Natural Link between Europe and Africa: 2.1 Billion Birds on Migration. Oikos 2009, 118, 624–626. [Google Scholar] [CrossRef]
- Packmor, F.; Klinner, T.; Woodworth, B.K.; Eikenaar, C.; Schmaljohann, H. Stopover departure decisions in songbirds: Do long-distance migrants depart earlier and more independently of weather conditions than medium-distance migrants? Mov. Ecol. 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Arnold, J.; Humer, A.; Heltai, M.; Murariu, D.; Spassov, N.; Hackländer, K. Current status and distribution of golden jackals Canis aureus in Europe. Mamm. Rev. 2012, 42, 1–11. [Google Scholar] [CrossRef]
- Cirovic, D.; Teodorovic, V.; Vasilev, D.; Markovic, M.; Cosic, N.; Dimitrijevic, M.; Klun, I.; Djurkovic-Djakovic, O. A large-scale study of the Trichinella genus in the golden jackal (Canis aureus) population in Serbia. Vet. Parasitol. 2015, 212, 253–256. [Google Scholar] [CrossRef]
- Gherman, C.M.; Mihalca, A.D. A synoptic overview of golden jackal parasites reveals high diversity of species. Parasit. Vectors 2017, 10, 419. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.M.; May, R.M. Coevolution of hosts and parasites. Parasitology 1982, 85 Pt. 2, 411–426. [Google Scholar] [CrossRef]
- Ben-Ami, F. The virulence-transmission relationship in an obligate killer holds under diverse epidemiological and ecological conditions, but where is the tradeoff? Ecol. Evol. 2017, 7, 11157–11166. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Escobar, M.; Calero-Bernal, R.; Regidor-Cerrillo, J.; Vallejo, R.; Benavides, J.; Collantes-Fernández, E.; Ortega-Mora, L.M. In vivo and in vitro models show unexpected degrees of virulence among Toxoplasma gondii type II and III isolates from sheep. Vet. Res. 2021, 52, 82. [Google Scholar] [CrossRef]



| Genotyping Loci | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome | VIII | IX | X | Ib | Plastid | III | V | VI | VIIa | ||||||
| Number | Origin | Isolate | Tissue | Lineage | Internal strain ID | AltSAG2 | BTUB | GRA6 | C22-8 | Apico | C29-2 | L358 | PK1 | CS3 | ToxoDB genotype/isolate ID |
| 1 | Multiple species | N | Blood, AF, Heart | III ARCH | various | III | III | III | III | III | III | III | III | III | ToxoDB#2 |
| 2 | Multiple species | Y | AF, Heart | II ARCH | various | II | II | II | II | II | II | II | II | II | ToxoDB#1 |
| 3 | Horse, human | Y | Heart | II/III, rec | EQ39, 123c20 | II * | III | III * | III | II | III | III | III * | II * | ToxoDB#54 |
| 4 | Horse | Y | Heart | III VAR | EQ40 | II | I | III | II | III | III | III | II | III | TgPiPr14 |
| 5 | Pigeon | Y | Heart | III VAR | G13 | III | III | III | II | III | III | III | II | III | No match |
| 6 | Chicken, red fox | Y | Heart | III VAR | K1, 061-13 | II * | II * | I * | II * | III | III | III | III * | III * | No match |
| 7 | Golden jackal, human | N | Heart, AF | II/III, rec | 125-16, 46c21 | II * | II * | II * | II | III * | - | - | III * | II | No match |
| 8 | Human | N | AF | II or III VAR | 25c19, 100c16 | III * | III * | II * | II | III | - | - | I * | II * | No match |
| 9 | Human | N | AF | III VAR | 182c19 | III | III | III | I | III | - | III | II | ToxoDB#21, #120, #123 (w/o CS3) | |
| 10 | Golden jackal | N | heart | II VAR | 051-16 | II | II | II | III | - | - | - | III | I | No match |
| 11 | Golden jackal | N | Heart | II VAR | 057-16, 061-16, 062-16 | II * | III * | III * | II * | II | - | - | II * | - | No match |
| 12 | Golden jackal | N | Heart | II or III | 075-16, 061-16, 062-16 | II * | III * | III * | II * | - | - | - | II * | III | No match |
| 13 | Red fox | N | Heart | II VAR | 068-16 | II | III | II | II | II | - | - | III | - | No match |
| 14 | Red fox, grey wolf | N | Heart | II VAR | 103-16 | II * | III * | II * | II * | - | - | - | II * | II | No match |
| 15 | Grey wolf | N | Heart | II or III | 020-16 | II | III | II | II | III | - | - | III | - | No match |
| 16 | Human | N | AF | II VAR | 107AF | II | II | II | I | - | - | - | III | II | No match |
| 17 | Human | N | AF | II VAR | 8c21 | II | - | II | III | III | - | - | III | II | No match |
| 18 | Horse, human | N | Heart | II/III, rec | EQ12, BAL | II * | II * | III * | - | III | - | - | II * | II | No match |
| 19 | Human | N | AF | III VAR | 138c18 | III | - | III | II | III | - | - | III | II | ToxoDB#116, #130, #163 #187 (w/o CS3) |
| 20 | Human | N | AF | III VAR | 108c16 | III | III | II | - | III | - | - | II | II | No match |
| 21 | Red fox, human | N | Heart | II VAR | 113-16, 158c18 | II | III | II * | - | - | - | - | I * | II * | ToxoDB#45, #142, #149, #150, #203 (w/o CS3) |
| 22 | Red fox, human | N | Heart, AF | III VAR | 124-16, 82c16 | III * | II * | III * | III | - | - | - | III * | - | No match |
| 23 | Golden jackal | N | Heart | II VAR | 023-15 | II | III | II | II | - | - | - | III | - | No match |
| 24 | Human | N | CSF | II VAR | 269L | III | II | III | II | - | - | - | II | - | No match |
| 25 | Human | N | AF | III VAR | 132c15 | III | - | III | - | III | - | - | II | II | No match |
| 26 | Human, red fox | N | AF | II or III | 97c18, 121-16 | II * | III * | II * | - | - | - | - | III | II | ToxoDB#136 (all markers), #93, #105, #107, #135, #136, #137, #139, #172, #173, #174, #202 (w/o CS3) |
| 27 | Human | N | AF | III VAR | 83c16 | III | II | II | III | - | - | - | III | - | No match |
| 28 | Human | N | AF | III VAR | 65c20 | III | - | I | III | II | - | - | III | - | No match |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzelac, A.; Klun, I.; Ćirković, V.; Bauman, N.; Bobić, B.; Štajner, T.; Srbljanović, J.; Lijeskić, O.; Djurković-Djaković, O. Toxoplasma gondii Genotypes Circulating in Serbia—Insight into the Population Structure and Diversity of the Species in Southeastern Europe, a Region of Intercontinental Strain Exchange. Microorganisms 2021, 9, 2526. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms9122526
Uzelac A, Klun I, Ćirković V, Bauman N, Bobić B, Štajner T, Srbljanović J, Lijeskić O, Djurković-Djaković O. Toxoplasma gondii Genotypes Circulating in Serbia—Insight into the Population Structure and Diversity of the Species in Southeastern Europe, a Region of Intercontinental Strain Exchange. Microorganisms. 2021; 9(12):2526. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms9122526
Chicago/Turabian StyleUzelac, Aleksandra, Ivana Klun, Vladimir Ćirković, Neda Bauman, Branko Bobić, Tijana Štajner, Jelena Srbljanović, Olivera Lijeskić, and Olgica Djurković-Djaković. 2021. "Toxoplasma gondii Genotypes Circulating in Serbia—Insight into the Population Structure and Diversity of the Species in Southeastern Europe, a Region of Intercontinental Strain Exchange" Microorganisms 9, no. 12: 2526. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms9122526