Rational Design for Enhanced Acyltransferase Activity in Water Catalyzed by the Pyrobaculum calidifontis VA1 Esterase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Gene Expression and Protein Purification
2.3. In Silico Methods
2.4. Site-Directed Mutagenesis
2.5. SDS-PAGE Analysis
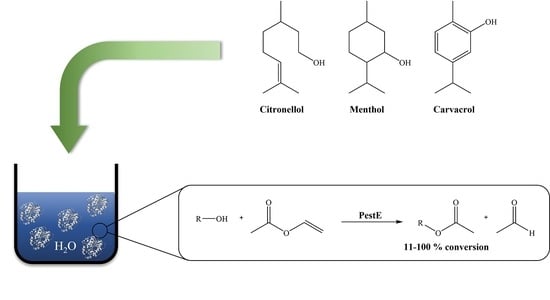
2.6. Biocatalytic Experiments
2.7. GC Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bornscheuer, U.T.; Kazlauskas, R.J. Hydrolases in Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Henke, E.; Pleiss, J.; Bornscheuer, U.T. Activity of lipases and esterases towards tertiary alcohols: Insights into structure- function relationships. Angew. Chem. Int. Ed. 2002, 41, 3211–3213. [Google Scholar] [CrossRef]
- Liese, A.; Seelbach, K.; Wandrey, C. (Eds.) Industrial Biotransformations, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Bornscheuer, U.T.; Kazlauskas, R.J. Catalytic promiscuity in biocatalysis: Using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. 2004, 43, 6032–6040. [Google Scholar] [CrossRef]
- Müller, H.; Becker, A.-K.; Palm, G.J.; Berndt, L.; Badenhorst, C.P.S.; Godehard, S.P.; Reisky, L.; Lammers, M.; Bornscheuer, U.T. Sequence-based prediction of promiscuous acyltransferase activity in hydrolases. Angew. Chem. Int. Ed. 2020, 59, 11607–11612. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.H.; Persson, M.; Bornscheuer, U.T. Enantioselective transesterification of a tertiary alcohol by lipase A from Candida antarctica. Tetrahedron Asymmetry 2002, 13, 2693–2696. [Google Scholar] [CrossRef]
- Wikmark, Y.; Humble, M.S.; Bäckvall, J.-E. Combinatorial library based engineering of Candida antarctica lipase A for enantioselective transacylation of sec-alcohols in organic solvent. Angew. Chem. Int. Ed. 2015, 54, 4284–4288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbst, D.; Peper, S.; Niemeyer, B. Enzyme catalysis in organic solvents: Influence of water content, solvent composition and temperature on Candida rugosa lipase catalyzed transesterification. J. Biotechnol. 2012, 162, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, E.; Szmulik, P.; Norin, T.; Hult, K. Hydrolysis and esterification with lipase from Candida cylindracea. Influence of the reaction conditions and acid moiety on the enantiomeric excess. Biocatalysis 1989, 2, 217–223. [Google Scholar] [CrossRef]
- Wu, X.Y.; Jääskeläinen, S.; Linko, Y.-Y. An investigation of crude lipases for hydrolysis, esterification and transesterification. Enzym. Microb. Technol. 1996, 19, 226–231. [Google Scholar] [CrossRef]
- van Rantwijk, F.; Hacking, M.A.P.J.; Sheldon, R.A. Lipase-catalyzed synthesis of carboxylic amides: Nitrogen nucleophiles as acyl acceptor. Monatsh. Chem. 2000, 131, 549–569. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Z.-L. Enzymatic synthesis of sugar fatty acid esters in ionic liquids. Catal. Sci. Technol. 2012, 2, 1767–1775. [Google Scholar] [CrossRef]
- Godehard, S.P.; Badenhorst, C.P.S.; Müller, H.; Bornscheuer, U.T. Protein engineering for enhanced acyltransferase activity, substrate scope, and selectivity of the Mycobacterium smegmatis acyltransferase MsAcT. ACS Catal. 2020, 10, 7552–7562. [Google Scholar] [CrossRef]
- Neungot, V.; Moulin, G.; Dubreucq, E.; Bigey, F. The lipase/acyltransferase from Candida prapsilosis—Molecular cloning and characterization of purified recombinant enzymes. Eur. J. Biochem. 2002, 269, 1734–1745. [Google Scholar]
- Mathews, I.; Soltis, M.; Saldajeno, M.; Ganshaw, G.; Sala, R.; Weyler, W.; Cervin, M.A.; Whited, G.; Bott, R. Structure of a novel enzyme that catalyzes acyl transfer to alcohols in aqueous conditions. Biochemistry 2007, 46, 8969–8979. [Google Scholar] [CrossRef] [PubMed]
- Godehard, S.P.; Müller, H.; Badenhorst, C.P.S.; Stanetty, C.; Suster, C.; Mihovilovic, M.D.; Bornscheuer, U.T. Efficient acylation of sugars and oligosaccharides in aqueous environment using engineered acyltransferases. ACS Catal. 2021, 11, 2831–2836. [Google Scholar] [CrossRef]
- Müller, H.; Godehard, S.P.; Palm, G.J.; Berndt, L.; Badenhorst, C.P.S.; Becker, A.-K.; Lammers, M.; Bornscheuer, U.T. Discovery and design of family VIII carboxylesterases as highly efficient acyltransferases. Angew. Chem. Int. Ed. 2021, 60, 2013–2017. [Google Scholar] [CrossRef]
- Reisky, L.; Srinivasamurthy, V.S.T.; Badenhorst, C.P.S.; Godehard, S.P.; Bornscheuer, U.T. A novel high-throughput assay enables the direct identification of acyltransferases. Catalysts 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Liu, J.; Anankanbil, S.; Chen, M.; Guo, Z.; Adams, J.P.; Snajdrova, R.; Li, Z. Amide synthesis via aminolysis of ester or acid with an intracellular lipase. ACS Catal. 2018, 8, 8856–8865. [Google Scholar] [CrossRef]
- Müller, J.; Sowa, M.A.; Dörr, M.; Bornscheuer, U.T. The acyltransferase activity of lipase CAL-A allows efficient fatty acid esters formation from plant oil even in an aqueous environment. Eur. J. Lipid Sci. Technol. 2015, 117, 1903–1907. [Google Scholar] [CrossRef]
- Land, H.; Hendil-Forssell, P.; Martinelle, M.; Berglund, P. One-pot biocatalytic amine transaminase/acyl transferase cascade for aqueous formation of amides from aldehydes or ketones. Catal. Sci. Technol. 2016, 6, 2897–2900. [Google Scholar] [CrossRef] [Green Version]
- Hotta, Y.; Ezaki, S.; Atomi, H.; Imanaka, T. Extremely stable and versatile carboxylesterase from a hyperthermophilic archaeon. Appl. Environ. Microbiol. 2002, 68, 3925–3931. [Google Scholar] [CrossRef] [Green Version]
- Kourist, R.; de María, P.D.; Bornscheuer, U.T. Enzymatic synthesis of optically active tertiary alcohols: Expanding the biocatalysis toolbox. ChemBioChem 2008, 9, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Palm, G.J.; Fernandez-Álvaro, E.; Bogdanović, X.; Bartsch, S.; Sczodrok, J.; Singh, R.K.; Böttcher, D.; Atomi, H.; Bornscheuer, U.T.; Hinrichs, W. The crystal structure of an esterase from the hyperthermophilic microorganism Pyrobaculum calidifontis VA1 explains its enantioselectivity. Appl. Environ. Microbiol. 2011, 91, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Darden, T.; Nabuurs, S.B.; Finkelstein, A.; Wriend, G. Making optimal use of empirical energy functions: Force-field parameterization in crystal space. Proteins 2004, 57, 678–683. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Kokkonen, P.; Bednar, D.; Pinto, G.; Prokop, Z.; Damborsky, J. Engineering enzymes access tunnels. Biotechnol. Adv. 2019, 37, 107386. [Google Scholar] [CrossRef]
- Rakels, J.L.L.; Straathof, A.J.J.; Heijnen, J.J. A simple method to determine the enantiomeric ratio in enantioselective biocatalysis. Enzym. Microb. Technol. 1993, 15, 1051–1056. [Google Scholar] [CrossRef]
- Kazemi, M.; Sheng, X.; Kroutil, W.; Himo, F. Computational study of Mycobacterium smegmatis acyl transferase reaction mechanism and specificity. ACS Catal. 2018, 8, 10698–10706. [Google Scholar] [CrossRef]
- Paravidino, M.; Hanefeld, U. Enzymatic acylation: Assessing the greenness of different acyl donors. Green Chem. 2011, 13, 2651–2657. [Google Scholar] [CrossRef] [Green Version]


| Time (min) | PestE_wt | PestE_H95A | PestE_I208A | PestE_N288F | ||||
|---|---|---|---|---|---|---|---|---|
| Conv (%) | %ee (E) | Conv (%) | %ee (E) | Conv (%) | %ee (E) | Conv (%) | %ee (E) | |
| 10 | 0 | - | 0 | - | 12 | 100 | 0 | - |
| 30 | 7 | 74 | 3 | 67 | 17 | 100 | 3 | 100 |
| 60 | 18 | 67 | 8 | 81 | 31 | 95 (59) | 9 | 97 |
| 120 | 32 | 59 (5) | 24 | 66 | 36 | 94 (55) | 20 | 89 |
| 180 | 37 | 58 (5) | 31 | 62 (6) | 38 | 94 (58) | 29 | 81 |
| 240 | 38 | 57 (5) | 28 | 66 | 36 | 95 (67) | 35 | 79 (13) |
| 480 | 32 | 68 (7) | 31 | 71 (8) | 24 | 100 | 44 | 80 (17) |
| 1440 | 19 | 80 | 9 | 67 | 13 | 100 | 38 | 79 (14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudt, A.; Terholsen, H.; Kaur, J.; Müller, H.; Godehard, S.P.; Itabaiana, I., Jr.; Leal, I.C.R.; Bornscheuer, U.T. Rational Design for Enhanced Acyltransferase Activity in Water Catalyzed by the Pyrobaculum calidifontis VA1 Esterase. Microorganisms 2021, 9, 1790. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms9081790
Staudt A, Terholsen H, Kaur J, Müller H, Godehard SP, Itabaiana I Jr., Leal ICR, Bornscheuer UT. Rational Design for Enhanced Acyltransferase Activity in Water Catalyzed by the Pyrobaculum calidifontis VA1 Esterase. Microorganisms. 2021; 9(8):1790. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms9081790
Chicago/Turabian StyleStaudt, Amanda, Henrik Terholsen, Jasmin Kaur, Henrik Müller, Simon P. Godehard, Ivaldo Itabaiana, Jr., Ivana C. R. Leal, and Uwe T. Bornscheuer. 2021. "Rational Design for Enhanced Acyltransferase Activity in Water Catalyzed by the Pyrobaculum calidifontis VA1 Esterase" Microorganisms 9, no. 8: 1790. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms9081790