Can Endocrine Dysfunction Be Reliably Tested in Aged Horses That Are Experiencing Pain?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses
2.2. Pain Assessment
2.2.1. Adrenocorticotropic Hormone (ACTH) and Cortisol Measurements
2.2.2. Control Examination
2.2.3. Statistics
3. Results
3.1. Horses
3.2. General Clinical Examination
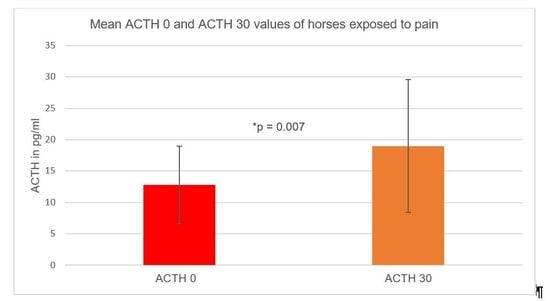
3.3. Adrenocorticotropic Hormone (ACTH) Concentration
3.3.1. Adrenocorticotropic Hormone 30 Minutes (ACTH 30) after Thyrotropin-Releasing Hormone (TRH) Stimulation Test
3.3.2. Pain Groups and Adrenocorticotropic Hormone (ACTH) Levels
3.4. Cortisol Concentration
3.5. Pain Groups and Cortisol Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGowan, T.W.; Pinchbeck, G.P.; McGowan, C.M. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet. J. 2013, 45, 74–79. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, D. Equine pituitary pars intermedia dysfunction. Vet. Clin. N. Am. Equine Pract. 2011, 27, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Schott, H.; Andrews, F.; Durham, A.; Frank, N.; Hart, K.; Kritchevsky, J.; McFarlane, D.; Tadros, L. Recommendations for the Diagnosis and Treatment of Pituitary Pars Intermedia Dysfunction (PPID). Equine Endocrinology Group. 2017. Available online: http://sites.tufts.edu/equineendogroup/ (accessed on 16 June 2020).
- Collins, S.N.; Pollitt, C.; Wylie, C.E.; Matiasek, K. Laminitic pain: Parallels with pain states in humans and other species. Vet. Clin. N. Am. Equine Pract. 2010, 26, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.T.; Jørgensen, A.J.R.; Beech, J. Evaluation of suspected pituitary pars intermedia dysfunction in horses with laminitis. J. Am. Vet. Med. Assoc. 2004, 224, 1123–1127. [Google Scholar] [CrossRef]
- Bussières, G.; Jacques, C.; Lainay, O.; Beauchamp, G.; Leblond, A.; Cadoré, J.L.; Desmaizieres, L.M.; Cuvelliez, S.G.; Troncy, E. Development of a composite orthopaedic pain scale in horses. Res. Vet. Sci. 2008, 85, 294–306. [Google Scholar] [CrossRef]
- Graubner, C.; Gerber, V.; Doherr, M.; Spadavecchia, C. Clinical application and reliability of a post abdominal surgery pain assessment scale (PASPAS) in horses. Vet. J. 2011, 188, 178–183. [Google Scholar] [CrossRef]
- Rietmann, R.; Stauffacher, M.; Bernasconi, P.; Auer, J.A.; Weishaupt, M.A. The association between heart rate, heart rate variability, endocrine and behavioural pain measures in horses suffering from laminitis. J. Vet. Med. A Phys. Path. Clin. Med. 2004, 51, 218–225. [Google Scholar] [CrossRef]
- Meier, A.; de Laat, M.; Pollitt, C.; Walsh, D.; McGree, J.; Reiche, D.B.; von Salis-Soglio, M.; Wells-Smith, L.; Mengeler, U.; Mesa Salas, D.; et al. A modified “Obel” method for the severity scoring of (endocrinopathic) equine laminitis. Peer J. 2019, 7, 7. [Google Scholar] [CrossRef]
- Beech, J.; Boston, R.; Lindborg, S.; Russell, G.E. Adrenocorticotropin concentration following administration of thyrotropin-releasing hormone in healthy horses and those with pituitary pars intermedia dysfunction and pituitary gland hyperplasia. J. Am. Vet. Med. Assoc. 2007, 231, 417–426. [Google Scholar] [CrossRef]
- Molony, V.; Kent, J.E. Assessment of acute pain in farm animals using behavioral and physiological measurements. J. Anim. Sci. 1997, 75, 266–272. [Google Scholar] [CrossRef]
- Durham, A.E. Endocrine disease in aged horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.E.; McGowan, C.M.; Fey, K.; Tamzali, Y.; van der Kolk, J.H. Pituitary pars intermedia dysfunction: Diagnosis and treatment. Equine Vet. Educ. 2014, 26, 216–223. [Google Scholar] [CrossRef]
- Secombe, C.J.; Bailey, S.R.; de Laat, M.A.; Hughes, K.J.; Stewart, A.J.; Sonis, J.M.; Tan, R. Equine pituitary pars intermedia dysfunction: Current understanding and recommendations from the Australian and New Zealand Equine Endocrine Group. Aust. Vet. J. 2018, 96, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Antakly, T.; Eisen, H.J. Immunocytochemical localization of glucocorticoid receptors in target cells. Endocrinology 1984, 115, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Antakly, T.; Sasaki, A.; Liotta, A.S.; Palkovits, M.; Krieger, D.T. Induced expression of the glucocorticoid receptor in the rat intermediate pituitary lobe. Science 1985, 229, 277–279. [Google Scholar] [CrossRef]
- Antakly, T.; Mercille, S.; Côté, J.P. Tissue-specific dopaminergic regulation of the glucocorticoid receptor in the rat pituitary. Endocrinology 1987, 120, 1558–1562. [Google Scholar] [CrossRef]
- Bertini, L.T.; Westphal, M.M.; Kloet, R.E.; Kiss, J.Z. Glucocorticoid receptor immunoreactivity in the rat intermediate lobe. J. Neuroendocr. 1989, 1, 465–471. [Google Scholar] [CrossRef]
- Seger, M.A.; van Eekelen, J.A.; Kiss, J.Z.; Burbach, J.P.; de Kloet, E.R. Stimulation of pro-opiomelanocortin gene expression by glucocorticoids in the denervated rat intermediate pituitary gland. Neuroendocrinology 1988, 47, 350–357. [Google Scholar] [CrossRef]
- Raekallio, M.; Taylor, P.M.; Bennett, R.C. Preliminary investigations of pain and analgesia assessment in horses administered phenylbutazone or placebo after arthroscopic surgery. Vet. Surg. 1997, 26, 150–155. [Google Scholar] [CrossRef]
- Mellor, D.; Stafford, K.; Todd, S.; Lowe, T.; Gregory, N.; Bruce, R.; Ward, R. A comparison of catecholamine and cortisol responses of young lambs and calves to painful husbandry procedures. Aust. Vet. J. 2002, 80, 228–233. [Google Scholar] [CrossRef]
- Pritchett, L.C.; Ulibarri, C.; Roberts, M.C.; Schneider, R.K.; Sellon, D.C. Identification of potential physiological and behavioral indicators of postoperative pain in horses after exploratory celiotomy for colic. Appl. Anim. Behav. Sci. 2003, 80, 31–43. [Google Scholar] [CrossRef]
- Ayala, I.; Martos, N.F.; Silvan, G.; Gutierez-Panizo, C.; Clavel, J.G.; Illera, J.C. Cortisol, adrenocorticotropic hormone, serotonin, adrenaline and noradrenaline serum concentrations in relation to disease and stress in the horse. Res. Vet. Sci. 2012, 93, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hada, T.; Onaka, T.; Takahashi, T.; Hiraga, A.; Yagi, K. Effects of novelty stress on neuroendocrine activities and running performance in thoroughbred horses. J. Neuroendocr. 2003, 15, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Fazio, E.; Medica, P.; Aronica, V.; Grasso, L.; Ferlazzo, A. Circulating beta-endorphin, adrenocorticotrophic hormone and cortisol levels of stallions before and after short road transport: Stress effect of different distances. Acta Vet. Scand. 2008, 50, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Schonbom, H.; Kassens, A.; Hopster-Iversen, C.; Klewitz, J.; Piechotta, M.; Martinsson, G.; Kissler, A.; Burger, D.; Sieme, H. Influence of transrectal and transabdominal ultrasound examination on salivary cortisol, heart rate, and heart rate variability in mares. Theriogenology 2015, 83, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Niinistö, K.E.; Korolainen, R.V.; Raekallio, M.R.; Mykkanen, A.K.; Koho, N.M.; Ruohoniemi, M.O.; Leppaluoto, J.; Poso, A.R. Plasma levels of heat shock protein 72 (HSP72) and beta-endorphin as indicators of stress, pain and prognosis in horses with colic. Vet. J. 2010, 184, 100–104. [Google Scholar] [CrossRef]
- Towns, T.J.; Stewart, A.J.; Hackett, E.; Zhong, Q.; Munstermann, A.; Wooldridge, A.A.; Funk, R.A.; Hewes, C.A. Cortisol and ACTH concentrations in ill horses throughout 6 days of hospitalization. J. Vet. Emerg. Crit. Care 2010, 20, A16–A17. [Google Scholar]
- Gold, J.R.; Divers, T.J.; Barton, M.H.; Lamb, S.V.; Place, N.J.; Mohammed, H.O.; Bain, F.T. Plasma adrenocorticotropin, cortisol, and adrenocorticotropin/cortisol ratios in septic and normal-term foals. J. Vet. Int. Med. 2007, 21, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Tennant, F.; Hermann, L. Normalization of serum cortisol concentration with opioid treatment of severe chronic pain. Pain Med. 2002, 3, 132–134. [Google Scholar] [CrossRef]
- Muhtz, C.; Rodriguez-Raecke, R.; Hinkelmann, K.; Möller-Bertram, T.; Kiefer, F.; Wiedemann, K.; May, A.; Otte, C. Cortisol response to experimental pain in patients with chronic low back pain and patients with major depression. Pain Med. 2013, 14, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Tennant, F. Adrenocorticotropin (ACTH) in chronic pain. J. Appl. Biobehav. Res. 2017, 22, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.L.; Irvine, C.H.; Ellis, M.J.; Donald, R.A. The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous blood from the horse. Endocrinology 1991, 128, 65–72. [Google Scholar] [CrossRef]
- Colahan, P.T.; Bailey, J.E.; Chou, C.C.; Johson, M.; Rice, B.L.; Jones, G.L.; Cheeks, J.P. Effect of flunixin meglumine on selected physiologic and performance parameters of athletically conditioned thoroughbred horses subjected to an incremental exercise stress test. Vet. Ther. 2002, 3, 37–48. [Google Scholar]
- Foreman, J.H.; Barange, A.; Lawrence, L.M.; Hungerford, L.L. Effects of single-dose intravenous phenylbutazone on experimentally induced, reversible lameness in the horse. J. Vet. Pharm. Ther. 2008, 31, 39–44. [Google Scholar] [CrossRef]
- Sellon, D.C.; Roberts, M.C.; Blikslager, A.T.; Ulibarri, C.; Papich, M.G. Effects of continuous rate intravenous infusion of butorphanol on physiologic and outcome variables in horses after celiotomy. J. Vet. Int. Med. 2004, 18, 555–563. [Google Scholar] [CrossRef]
- Aloisi, A.M.; Buonocore, M.; Merlo, L.; Galandra, C.; Sotiu, A.; Bacchella, L.; Ungaretti, M.; Demartini, L.; Bonezzi, C. Chronic pain therapy and hypothalamic-pituitary-adrenal axis impairment. Psychoneuroendocrinology 2011, 36, 1032–1039. [Google Scholar] [CrossRef]
- Taylor, P.M. Equine stress responses to anaesthesia. Br. J. Anaest. 1989, 63, 702–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creighton, C.M.; Lemke, K.A.; Lamont, L.A.; Horney, B.S.; Doyle, A.J. Comparison of the effects of xylazine bolus versus medetomidine constant rate infusion on the stress response, urine production, and anesthetic recovery characteristics in horses anesthetized with isoflurane. J. Am. Vet. Med. Assoc. 2012, 240, 998–1002. [Google Scholar] [CrossRef]
- Donaldson, L.L.; Dunlop, G.S.; Holland, M.S.; Burton, B.A. The recovery of horses from inhalant anesthesia: A comparison of halothane and isoflurane. Vet. Surg. 2000, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cook, V.L.; Blikslager, A.T. Use of systemically administered lidocaine in horses with gastrointestinal tract disease. J. Am. Vet. Med. Assoc. 2008, 232, 1144–1148. [Google Scholar] [CrossRef]
- Sanchez, L.C.; Robertson, S.A. Pain control in horses: What do we really know? Equine Vet. J. 2014, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, S.A.E.; De Vries, A.; Raekallio, M.R.; Mykkanen, A.K.; Palviainen, M.J.; Sankari, S.M.; Vainio, O.M. Changes in energy metabolism, and levels of stress-related hormones and electrolytes in horses after intravenous administration of romifidine and the peripheral alpha-2 adrenoceptor antagonist vatinoxan. Acta Vet. Scand. 2018, 60, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raekallio, M.; Leino, A.; Vainio, O.; Scheinin, M. Sympatho-adrenal activity and the clinical sedative effect of detomidine in horses. Equine Vet. J. 1992, 24, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Carroll, G.L.; Matthews, N.S.; Hartsfield, S.M.; Slater, M.R.; Champney, T.H.; Erickson, S.W. The effect of detomidine and its antagonism with tolazoline on stress-related hormones, metabolites, physiologic responses, and behavior in awake ponies. Vet. Surg. 1997, 26, 69–77. [Google Scholar] [CrossRef]
- Van Loon, J.P.; Back, W.; Hellebrekers, L.J.; van Weeren, P.R. Application of a composite pain scale to objectively monitor horses with somatic and visceral pain under hospital conditions. J. Equine Vet. Sci. 2010, 30, 641–649. [Google Scholar] [CrossRef]
- van Loon, J.P.; Jonckheer-Sheehy, V.S.; Back, W.; van Weeren, P.; Hellebrekers, L.J. Monitoring equine visceral pain with a composite pain scale score and correlation with survival after emergency gastrointestinal surgery. Vet. J. 2014, 200, 109–115. [Google Scholar] [CrossRef]
- Ashley, F.H.; Waterman-Pearson, A.E.; Whay, H.R. Behavioral assessment of pain in horses and donkeys: Application to clinical practice and future studies. Equine Vet. J. 2005, 37, 565–575. [Google Scholar] [CrossRef]
- De Grauw, J.C.; van Loon, J.P. Systematic pain assessment in horses. Vet. J. 2016, 209, 14–22. [Google Scholar] [CrossRef]
- May, A. Evaluierung von Stressparametern beim Pferd im Zusammenhang mit dem Klinikaufenthalt [Evaluation of Stress Parameters in Horses in Connection with the Stay in Hospital]. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2007. [Google Scholar]
- McFarlane, D.; Beech, J.; Cribb, A. Alpha-melanocyte stimulating hormone release in response to thyrotropin releasing hormone in healthy horses, horses with pituitary pars intermedia dysfunction and equine pars intermedia explants. Domest. Anim. Endocrinol. 2006, 30, 276–288. [Google Scholar] [CrossRef]
- France, R.D.; Krishan, K.R.; Goli, V.; Manepalli, A.N.; Dickson, L. Preliminary study of thyrotrophin releasing hormone stimulation test in chronic low back pain patients. Pain 1986, 27, 51–55. [Google Scholar] [CrossRef]
- Raekallio, M.; Taylor, P.M.; Bloomfield, M. A comparison of methods for evaluation of pain and distress after orthopaedic surgery in horses. J. Vet. Anesthes. 1997, 24, 17–20. [Google Scholar] [CrossRef]
- Hood, D.M. Laminitis as a systemic disease. Vet. Clin. N. Am. Equine Pract. 1999, 15, 481–494. [Google Scholar] [CrossRef]
- Tennant, F. How to use adrenocorticotropin as a biomarker in pain management. Pract. Pain Manag. 2012, 12, 62–66. [Google Scholar]
- Mair, T.S.; Sherlock, C.E.; Boden, L.A. Serum cortisol concentrations in horses with colic. Vet. J. 2014, 201, 370–377. [Google Scholar] [CrossRef]
- Toribio, R.E.; Reed, S.M.; Warwick, M.B.; Sellon, D.C. Equine Internal Medicine, 4th ed.; Elsevier: St. Louis, MO, USA, 2018; p. 1063. [Google Scholar]
- Himler, M.; Hurcombe, S.D.; Griffin, A.; Barsnick, R.J.; Rathgeber, R.A.; MacGillivray, K.C.; Toribio, R.E. Presupmtive non-thyroidal illness syndrome in critically ill foals. Equine Vet. J. Suppl. 2012, 44, 43–47. [Google Scholar] [CrossRef]
- Breuhaus, B.A. Thyroid function and dysfunction in term and premature equine neonates. J. Vet. Intern. Med. 2014, 28, 1301–1309. [Google Scholar] [CrossRef] [Green Version]
- Hilderbran, A.C.; Breuhaus, B.A.; Refsal, K.R. Nonthyroidal illness syndrome in adult horses. J. Vet. Intern. Med. 2014, 28, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Strittmatter, M.; Bianchi, O.; Ostertag, D.; Grauer, M.; Paulus, C.; Fischer, C.; Meyer, S. Funktionsstörung der hypothalamisch-hypophysär-adrenalen Achse bei Patienten mit akuten, chronischen und intervallartigen Schmerzsyndromen [Dysfunction of the hypothalamic-pituitary-adrenal axis in patients with acute, chronic and interval pain syndromes]. Schmerz 2005, 19, 109–116. [Google Scholar] [CrossRef]
- Dickens, M.J.; Romero, L.M. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen. Comp. Endocrinol. 2013, 191, 177–189. [Google Scholar] [CrossRef]
- Irvine, C.H.; Alexander, S.L. Factors affecting the circadian rhythm in plasma cortisol concentrations in the horse. Domest. Anim. Endocrinol. 1994, 11, 227–238. [Google Scholar] [CrossRef]
- Mills, P.C.; Ng, J.C.; Kramer, H.; Auer, D.E. Stress response to chronic inflammation in the horse. Equine Vet. J. 1997, 29, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.A. The use of cortisol for the objective assessment of stress in animals: Pros and cons. Vet. J. 2012, 192, 137–139. [Google Scholar] [CrossRef]
- Beech, J.; Garcia, M. Hormonal response to thyrotropin-releasing hormone in healthy horses and in horses with pituitary adenoma. Am. J. Vet. Res. 1985, 46, 1941–1943. [Google Scholar] [PubMed]
- Beech, J.; Boston, R.; Lindborg, S. Comparison of cortisol and ACTH responses after administration of thyrotropin releasing hormone in normal horses and those with pituitary pars intermedia dysfunction. J. Vet. Int. Med. 2011, 25, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Eiler, H.; Oliver, J.W.; Andrews, F.M.; Fecteau, K.A.; Green, E.M.; McCracken, M. Results of a combined dexamethasone suppression thyrotropin-releasing hormone stimulation test in healthy horses and horses suspected to have a pars intermedia pituitary adenoma. J. Am. Vet. Med. Assoc. 1997, 211, 79–81. [Google Scholar]
| Parameter | Importance/Symptoms | Score |
|---|---|---|
| Behavior Movements (spontaneous) | Horse stands relaxed or shows calm movements. | 0 |
| Reduced movement or mild agitation. | 1 | |
| Reluctance to move or moderate agitation. | 2 | |
| Does not move, appears introverted or makes uncontrollable forward movements. | 3 | |
| Appetite Feed intake | Eats hay willingly (may wear a muzzle). | 0 |
| Eats hay reluctantly. | 1 | |
| Shows little interest in hay, eats only a few stalks or takes hay in the mouth but does not chew or swallow. | 2 | |
| Shows no interest in hay and does not eat any. | 3 | |
| Sweating | No sweating, dry coat. | 0 |
| Coat feels clammy. | 1 | |
| Coat feels damp, beads of sweat visible. | 2 | |
| Heavy sweating, sweat runs off the body. | 3 | |
| Heart rate | 22–44 | 0 |
| 45–52 | 1 | |
| 53–60 | 2 | |
| >60 | 3 | |
| Respiratory rate | <20 | 0 |
| 20–24 | 1 | |
| 25–30 | 2 | |
| >30 | 3 | |
| Internal body temperature | 36.9–38.5 °C | 0 |
| 36.4–36.9 °C or 38.5–39.0 °C | 1 | |
| 35.9–36.4 °C or 39.0–39.5 °C | 2 | |
| 35.4–35.9 °C or 39.5–40.0 °C | 3 |
| Parameter | Importance/Symptoms | Score |
|---|---|---|
| Gut sounds | Normal motility (++) | 0 |
| Reduced motility (+) | 1 | |
| No motility (-) | 2 | |
| Hypermotility (+++) | 3 | |
| Kicking to the stomach | Horse stands still, no kicking to the stomach. | 0 |
| Occasionally kicks against the stomach. (1–2 times in 5 min) | 1 | |
| Kicks regularly against the stomach. (3–4 times in 5 min) | 2 | |
| Kicks excessively against the stomach. (>5 times in 5 min) | 3 | |
| Pawing | Horse stands still, no scratching. | 0 |
| Occasional scratching. (1–2 times in 5 min) | 1 | |
| Regular scratching. (3–4 times in 5 min) | 2 | |
| Excessive scratching. (>5 times in 5 min) | 3 | |
| Head movements | No sign of discomfort, head is mainly held straight in front of the body. | 0 |
| Intermittent, lateral or vertical head movements, occasionally looking at the flank (1–2 times in 5 min) and/or lifting the lips (1–2 times in 5 min). | 1 | |
| Intermittent, violent, lateral or vertical head movements, looking regularly at the flank (3–4 times in 5 min) and/or lifting the lips (3–4 times in 5 min). | 2 | |
| Continuous head movements, looking excessively at the flank (>5 times in 5 min) and/or lifting the lips (>5 times in 5 min). | 3 | |
| Lying down, rolling | Horse stands quietly in the box. | 0 |
| Occasionally lying down. | 1 | |
| Regularly lying down and getting up again, rolling. | 2 | |
| Horse repeatedly throws itself down uncontrollably and rolls on the ground. | 3 |
| Parameter | Importance/Symptoms | Score |
|---|---|---|
| Posture | Normal movements, stands still with even weight distribution on all four limbs. | 0 |
| Occasional weight shift with temporary relieving posture, slight muscle tremor. | 1 | |
| Abnormal weight distribution, relieves a limb. | 2 | |
| Muscle tremor, exhaustion, sawhorse posture/arched back. | 3 | |
| Obel scale | No abnormalities in movement. | 0 |
| At rest, constant shifting of weight from one leg to the other. No lameness at walking pace but the horse shows a shortened, stiff walk at a trot. (Obel grade I) | 1 | |
| Horse walks willingly at walking pace but with a noticeably shortened and stiff gait. A limb can be lifted without any problems. (Obel grade II) | 2 | |
| Horse moves only reluctantly. A limb is difficult or impossible to pick up. (Obel Grade III) | 3 | |
| Horse refuses to move. Only moves when forced to. (Obel Grade IV) | 4 | |
| Pulsation of the Aa. digitalis palmaris lateralis and medialis | Physiological pulsation. | 0 |
| Slightly increased pulsation. | 1 | |
| Moderately increased pulsation. | 2 | |
| Highly increased pulsation. | 3 | |
| Reaction to the hoof pincers | No retraction of the limb. | 0 |
| Retraction of the limb when strong pressure is applied with the hoof pincers. | 1 | |
| Retraction of the limb, even under slight pressure. | 2 | |
| Retraction of the limb, even when pressure is exerted only with the hand. | 3 |
| Parameter | Importance/Symptoms | Score |
|---|---|---|
| Posture | Normal movements, stands still with even weight distribution on all four limbs. | 0 |
| Occasional weight shift with temporary relieving posture, slight muscle tremor. | 1 | |
| Abnormal weight distribution, relieves a limb. | 2 | |
| Muscle tremor, exhaustion, sawhorse posture/arched back. | 3 | |
| Degree of lameness | No lameness discernible. (Grade 0/5) | 0 |
| Lameness is difficult to detect and not continuously visible. (Grade 1/5) | 1 | |
| The lameness is difficult to detect on a straight line at walking pace and trot. Under certain conditions, however, it can be continuously detected (e.g., on the circle, on hard ground). (Grade 2/5) | 2 | |
| Lameness continuously visible at the trot, under all conditions. (Grade 3/5) | 3 | |
| Severe lameness, obvious at a walking pace. (Grade 4/5) | 4 | |
| Severe lameness, only short-term loading of the limb in motion and/or at rest or complete relief of the limb. (Grade 5/5) | 5 | |
| Reaction when lifting the contralateral limb | Contralateral limb can be lifted without problems. | 0 |
| Contralateral limb can only be lifted with difficulty and for a short time. | 1 | |
| Contralateral limb cannot be lifted at all. | 2 |
| Percentile Ranks (%) | Significance |
|---|---|
| 0–20 | Nonexistent |
| 21–40 | Slight |
| 41–60 | Moderate |
| 61–80 | High |
| 81–100 | Extreme |
| Horse (Number) | Disease State | Pain Score (%) | Medication (Hours between Administration and Blood Sampling) | ACTH 0 (pg/mL) | ACTH 30 (pg/mL) | Cortisol 0 (ng/mL) | Cortisol 30 (ng/mL) |
|---|---|---|---|---|---|---|---|
| 1 | Orthopedic | 50 | Phenylbutazone (3.5) Amoxicillin (3) | 6.5 | 8.64 | 27 | - |
| 2 * | Orthopedic | 25 | Phenylbutazone (7.5) | 6.8 | 10.2 | 29 | 51.7 |
| 3 | Colic | 55.5 | Amoxicillin (6.25) Heparin (6.25) Metamizole (4.25) Flunixin (3.25) Lidocaine (3.25) | 18.6 | 22.7 | 103 | 116 |
| 4 | Orthopedic | 35.71 | Amoxicillin (7) Gentamicin (7) Flunixin (7) L-Polamivet (4) | 9.1 | 15.1 | 21 | 31.9 |
| 5 | Orthopedic | 42.85 | Phenylbutazone (6.5) | 9.4 | 15.2 | 23 | 53.3 |
| 6 * | Orthopedic | 39.28 | Amoxicillin (6) Gentamycin (6) Flunixin (6) | 12 | 25.8 | 67 | 116 |
| 7 * | Laminitis | 25.8 | Phenylbutazone (6.5) | 14.3 | 33.4 | 29 | 41.3 |
| 8 * | Laminitis | 25.8 | - | 24.8 | 40.2 | 30 | 43.5 |
| 9 | Orthopedic | 25 | Phenybutazone (5.75) | 18.5 | 29.4 | 25 | 45.5 |
| 10 * | Laminitis | 48.38 | Flunixin (5.75) Heparin (5.75) | 7.4 | 2.5 | 60 | 54.4 |
| 11 * | Orthopedic | 28.57 | Amoxicillin (5.25) Gentamycin (5.25) Phenylbutazone (5.25) Omeprazole (5.25) | 17.9 | 13.1 | 31 | 35.2 |
| 12 * | Laminitis | 35.48 | Flunixin (5.25) Heparin (5.25) | 5 | 5.5 | 58 | 50.8 |
| 13 * | Orthopedic | 32.14 | Amoxicillin (5.75) Dembrexin (5.75) Flunixin (3.75) | 21.62 | 18.71 | 68.5 | 62.4 |
| 14 | Colic | 33.33 | Amoxicillin (7.25) Gentymycin (7.25) Heparin (7.25) Metamizole (1.25) Xylazin (1.25) Butorphanol (0.75) Flunixin (0.75) | 6.81 | 18.3 | 89.5 | 77 |
| 15 * | Orthopedic | 46.42 | - | 12.81 | 26.2 | 15.2 | 30.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehlen, H.; Jaburg, N.; Merle, R.; Winter, J. Can Endocrine Dysfunction Be Reliably Tested in Aged Horses That Are Experiencing Pain? Animals 2020, 10, 1426. https://0-doi-org.brum.beds.ac.uk/10.3390/ani10081426
Gehlen H, Jaburg N, Merle R, Winter J. Can Endocrine Dysfunction Be Reliably Tested in Aged Horses That Are Experiencing Pain? Animals. 2020; 10(8):1426. https://0-doi-org.brum.beds.ac.uk/10.3390/ani10081426
Chicago/Turabian StyleGehlen, Heidrun, Nina Jaburg, Roswitha Merle, and Judith Winter. 2020. "Can Endocrine Dysfunction Be Reliably Tested in Aged Horses That Are Experiencing Pain?" Animals 10, no. 8: 1426. https://0-doi-org.brum.beds.ac.uk/10.3390/ani10081426