Seroprevalence of Major Pasture-Borne Parasitoses (Gastrointestinal Nematodes, Liver Flukes and Lungworms) in German Dairy Cattle Herds, Association with Management Factors and Impact on Production Parameters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm Selection, Questionnaire and Farm Visits
2.2. Breed Information and Milk Production Parameters
2.3. BTM Sampling
2.4. ELISA Analyses
2.5. Data Analyses
3. Results
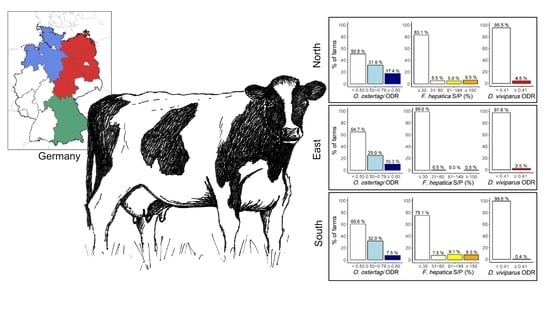
3.1. Regional and Annual Patterns of Seroprevalence
3.2. Breed Differences
3.3. Association of Seroprevalence with Management Factors
3.4. Association of Seropositivity Categories with BCS and Herd Productivity Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlier, J.; van der Voort, M.; Kenyon, F.; Skuce, P.; Vercruysse, J. Chasing helminths and their economic impact on farmed ruminants. Trends Parasitol. 2014, 30, 361–367. [Google Scholar] [CrossRef]
- Agneessens, J.; Claerebout, E.; Dorny, P.; Borgsteede, F.H.M.; Vercruysse, J. Nematode parasitism in adult dairy cows in Belgium. Vet. Parasitol. 2000, 90, 83–92. [Google Scholar] [CrossRef]
- Bellet, C.; Green, M.J.; Vickers, M.; Forbes, A.; Berry, E.; Kaler, J. Ostertagia spp., rumen fluke and liver fluke single- and poly-infections in cattle: An abattoir study of prevalence and production impacts in England and Wales. Prev. Vet. Med. 2016, 132, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Rehbein, S.; Visser, M.; Winter, R. Helminth infection in cattle from Schleswig-Holstein (Germany) after one grazing season. Berl. Muench. Tieraerztl. Wochenschr. 2003, 116, 41–44. [Google Scholar]
- Charlier, J.; Claerebout, E.; Duchateau, L.; Vercruysse, J. A survey to determine relationships between bulk tank milk antibodies against Ostertagia ostertagi and milk production parameters. Vet. Parasitol. 2005, 129, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Penedo, I.; Höglund, J.; Fall, N.; Emanuelson, U. Exposure to pasture borne nematodes affects individual milk yield in Swedish dairy herds. Vet. Parasitol. 2012, 188, 93–98. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Brügemann, K.; König, S.; Strube, C. Patent gastrointestinal nematode infections in organically and conventionally pastured dairy cows and their impact on individual milk and fertility parameters. Vet. Parasitol. 2017, 245, 119–127. [Google Scholar] [CrossRef]
- May, K.; Brügemann, K.; König, S.; Strube, C. Patent infections with Fasciola hepatica and paramphistomes (Calicophoron daubneyi) in dairy cows and association of fasciolosis with individual milk production and fertility parameters. Vet. Parasitol. 2019, 267, 32–41. [Google Scholar] [CrossRef]
- Köstenberger, K.; Tichy, A.; Bauer, K.; Pless, P.; Wittek, T. Associations between fasciolosis and milk production, and the impact of anthelmintic treatment in dairy herds. Parasitol. Res. 2017, 116, 1981–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, G.; Braun, U.; Deplazes, P.; Torgerson, P.R. Estimating the financial losses due to bovine fasciolosis in Switzerland. Vet. Rec. 2005, 157, 188–193. [Google Scholar] [CrossRef]
- May, K.; Bohlsen, E.; König, S.; Strube, C. Fasciola hepatica seroprevalence in Northern German dairy herds and associations with milk production parameters and milk ketone bodies. Vet. Parasitol. 2020, 277, 109016. [Google Scholar] [CrossRef]
- Holzhauer, M.; van Schaik, G.; Saatkamp, H.W.; Ploeger, H.W. Lungworm outbreaks in adult dairy cows: Estimating economic losses and lessons to be learned. Vet. Rec. 2011, 169, 494. [Google Scholar] [CrossRef]
- May, K.; Brügemann, K.; König, S.; Strube, C. The effect of patent Dictyocaulus viviparus (re)infections on individual milk yield and milk quality in pastured dairy cows and correlation with clinical signs. Parasit. Vectors 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Dank, M.; Holzhauer, M.; Veldhuis, A.; Frankena, K. Association between Dictyocaulus viviparus status and milk production parameters in Dutch dairy herds. J. Dairy Sci. 2015, 98, 7741–7747. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.; Ghebretinsae, A.; Meyns, T.; Czaplicki, G.; Vercruysse, J.; Claerebout, E. Antibodies against Dictyocaulus viviparus major sperm protein in bulk tank milk: Association with clinical appearance, herd management and milk production. Vet. Parasitol. 2016, 232, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bennema, S.; Vercruysse, J.; Claerebout, E.; Schnieder, T.; Strube, C.; Ducheyne, E.; Hendrickx, G.; Charlier, J. The use of bulk-tank milk ELISAs to assess the spatial distribution of Fasciola hepatica, Ostertagia ostertagi and Dictyocaulus viviparus in dairy cattle in Flanders (Belgium). Vet. Parasitol. 2009, 165, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.B.; Vercruysse, J.; Charlier, J. A survey of the exposure to Ostertagia ostertagi in dairy cow herds in Europe through the measurement of antibodies in milk samples from the bulk tank. Vet. Parasitol. 2008, 157, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Bennema, S.C.; Vercruysse, J.; Morgan, E.; Stafford, K.; Höglund, J.; Demeler, J.; von Samson-Himmelstjerna, G.; Charlier, J. Epidemiology and risk factors for exposure to gastrointestinal nematodes in dairy herds in northwestern Europe. Vet. Parasitol. 2010, 173, 247–254. [Google Scholar] [CrossRef]
- Fanke, J.; Charlier, J.; Steppin, T.; von Samson-Himmelstjerna, G.; Vercruysse, J.; Demeler, J. Economic assessment of Ostertagia ostertagi and Fasciola hepatica infections in dairy cattle herds in Germany using Paracalc®. Vet. Parasitol. 2017, 240, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kuerpick, B.; Conraths, F.J.; Staubach, C.; Fröhlich, A.; Schnieder, T.; Strube, C. Seroprevalence and GIS-supported risk factor analysis of Fasciola hepatica infections in dairy herds in Germany. Parasitology 2013, 140, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Schunn, A.-M.; Conraths, F.J.; Staubach, C.; Fröhlich, A.; Forbes, A.; Schnieder, T.; Strube, C. Lungworm infections in German dairy cattle herds—Seroprevalence and GIS-supported risk factor analysis. PLoS ONE 2013, 8, e74429. [Google Scholar] [CrossRef] [Green Version]
- Von Keyserlingk, M.A.G.; Amorim Cestari, A.; Franks, B.; Fregonesi, J.A.; Weary, D.M. Dairy cows value access to pasture as highly as fresh feed. Sci. Rep. 2017, 7, 44953. [Google Scholar] [CrossRef]
- Weinrich, R.; Kühl, S.; Zühlsdorf, A.; Spiller, A. Consumer attitudes in Germany towards different dairy housing systems and their implications for the marketing of pasture raised milk. Int. Food Agribus. Manag. Rev. 2014, 17, 205–222. [Google Scholar] [CrossRef]
- May, K.; Brügemann, K.; Yin, T.; Scheper, C.; Strube, C.; König, S. Genetic line comparisons and genetic parameters for endoparasite infections and test-day milk production traits. J. Dairy Sci. 2017, 100, 7330–7344. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.R.; Charlier, J.; Hendrickx, G.; Biggeri, A.; Catalan, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Müller, E.; Van Dijk, J.; Kenyon, F.; et al. Global change and helminth infections in grazing ruminants in Europe: Impacts, trends and sustainable solutions. Agriculture 2013, 3, 484–502. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J.; Sargison, N.D.; Kenyon, F.; Skuce, P.J. Climate change and infectious disease: Helminthological challenges to farmed ruminants in temperate regions. Animal 2010, 4, 377–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, J.; Ghebretinsae, A.H.; Levecke, B.; Ducheyne, E.; Claerebout, E.; Vercruysse, J. Climate-driven longitudinal trends in pasture-borne helminth infections of dairy cattle. Int. J. Parasitol. 2016, 46, 881–888. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; van Dijk, J. Spatiotemporal trends in cattle lungworm disease (Dictyocaulus viviparus) in Great Britain from 1975 to 2014. Vet. Rec. 2020, 186, 642. [Google Scholar] [CrossRef] [Green Version]
- Merle, R.; Busse, M.; Rechter, G.; Meer, U. Epidemiologie-Regionalisierung Deutschlands anhand landwirtschaftlicher Strukturdaten. Berl. Muench. Tieraerztl. Wochenschr. 2012, 125, 52–59. [Google Scholar] [CrossRef]
- PraeRi. Animal Health, Hygiene and Biosecurity in German Dairy Cow Operations—A Prevalence Study (PraeRi); Final Report. Available online: https://ibei.tiho-hannover.de/praeri/pages/69#_AB (accessed on 9 April 2021).
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Metzner, M.; Heuwieser, W.; Klee, W. Die Beurteilung der Körperkondition (body condition scoring) im Herdenmanagement. Prakt. Tierarzt 1993, 74, 991–998. [Google Scholar]
- Oehm, A.W.; Jensen, K.C.; Tautenhahn, A.; Mueller, K.-E.; Feist, M.; Merle, R. Factors associated with lameness in tie stall housed dairy cows in South Germany. Front. Vet. Sci. 2020, 7, 601640. [Google Scholar] [CrossRef]
- Nelson, A.J. On-farm nutrition diagnostics: Nutrition management involvement opportunities for dairy practicioners. In Proceedings of the Twenty-Ninth Annual Conference of the American Association of Bovine Practitioners, San Diego, CA, USA, 12–14 September 1996; pp. 76–85. [Google Scholar] [CrossRef]
- Kielland, C.; Ruud, L.E.; Zanella, A.J.; Østerås, O. Prevalence and risk factors for skin lesions on legs of dairy cattle housed in freestalls in Norway. J. Dairy Sci. 2009, 92, 5487–5496. [Google Scholar] [CrossRef] [Green Version]
- Kielland, C.; Skjerve, E.; Østerås, O.; Zanella, A.J. Dairy farmer attitudes and empathy toward animals are associated with animal welfare indicators. J. Dairy Sci. 2010, 93, 2998–3006. [Google Scholar] [CrossRef] [Green Version]
- Cook, N.B.; Hess, J.P.; Foy, M.R.; Bennett, T.B.; Brotzman, R.L. Management characteristics, lameness, and body injuries of dairy cattle housed in high-performance dairy herds in Wisconsin. J. Dairy Sci. 2016, 99, 5879–5891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Leach, K.A.; Dippel, S.; Huber, J.; March, S.; Winckler, C.; Whay, H.R. Assessing lameness in cows kept in tie-stalls. J. Dairy Sci. 2009, 92, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Schunn, A.-M.; Forbes, A.; Schnieder, T.; Strube, C. Validation of a Dictyocaulus viviparus MSP-ELISA and cut-off adjustment in a one-year longitudinal field study in dairy cattle herds. Vet. Parasitol. 2012, 189, 291–298. [Google Scholar] [CrossRef]
- Charlier, J.; Camuset, P.; Claerebout, E.; Courtay, B.; Vercruysse, J. A longitudinal survey of anti-Ostertagia ostertagi antibody levels in individual and bulk tank milk in two dairy herds in Normandy. Res. Vet. Sci. 2007, 83, 194–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, J.; Duchateau, L.; Claerebout, E.; Vercruysse, J. Predicting milk-production responses after an autumn treatment of pastured dairy herds with eprinomectin. Vet. Parasitol. 2007, 143, 322–328. [Google Scholar] [CrossRef]
- Von Holtum, C.; Strube, C.; Schnieder, T.; von Samson-Himmelstjerna, G. Development and evaluation of a recombinant antigen-based ELISA for serodiagnosis of cattle lungworm. Vet. Parasitol. 2008, 151, 218–226. [Google Scholar] [CrossRef]
- Fiedor, C.; Strube, C.; Forbes, A.; Buschbaum, S.; Klewer, A.-M.; von Samson-Himmelstjerna, G.; Schnieder, T. Evaluation of a milk ELISA for the serodiagnosis of Dictyocaulus viviparus in dairy cows. Vet. Parasitol. 2009, 166, 255–261. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.5.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dönges, J. Entwicklungs- und Lebensdauer von Metacercarien. Z. Parasitenkd. 1969, 31, 340–366. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lucas, H.C., Jr.; Shmueli, G. Research commentary—Too big to fail: Large samples and the p-value problem. Inf. Syst. Res. 2013, 24, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Frey, C.F.; Eicher, R.; Raue, K.; Strube, C.; Bodmer, M.; Hentrich, B.; Gottstein, B.; Marreros, N. Apparent prevalence of and risk factors for infection with Ostertagia ostertagi, Fasciola hepatica and Dictyocaulus viviparus in Swiss dairy herds. Vet. Parasitol. 2018, 250, 52–59. [Google Scholar] [CrossRef]
- Kowalczyk, S.J.; Czopowicz, M.; Weber, C.N.; Müller, E.; Nalbert, T.; Bereznowski, A.; Kaba, J. Herd-level seroprevalence of Fasciola hepatica and Ostertagia ostertagi infection in dairy cattle population in the central and northeastern Poland. BMC Vet. Res. 2018, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.C.; Knox, J.W.; Loyacano, A.F. Epidemiology of Ostertagia ostertagi in weaner-yearling cattle. Vet. Parasitol. 1993, 46, 313–324. [Google Scholar] [CrossRef]
- Ducheyne, E.; Charlier, J.; Vercruysse, J.; Rinaldi, L.; Biggeri, A.; Demeler, J.; Brandt, C.; de Waal, T.; Selemetas, N.; Hoglund, J.; et al. Modelling the spatial distribution of Fasciola hepatica in dairy cattle in Europe. Geospat. Health 2015, 9, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Hari, V.; Rakovec, O.; Markonis, Y.; Hanel, M.; Kumar, R. Increased future occurrences of the exceptional 2018–2019 Central European drought under global warming. Sci. Rep. 2020, 10, 12207. [Google Scholar] [CrossRef]
- Koch, S. Untersuchungen zur Verbreitung von Fasciola hepatica im Bayerischen Milchviehbestand. Ph.D. Thesis, Ludwig-Maximilians-Universität, Munich, Germany, 2005. [Google Scholar]
- Strube, C.; Springer, A.; Schunn, A.-M.; Forbes, A.B. Serological lessons from the bovine lungworm Dictyocaulus viviparus: Antibody titre development is independent of the infection dose and reinfection shortens seropositivity. Vet. Parasitol. 2017, 242, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bloemhoff, Y.; Forbes, A.; Good, B.; Morgan, E.; Mulcahy, G.; Strube, C.; Sayers, R. Prevalence and seasonality of bulk milk antibodies against Dictyocaulus viviparus and Ostertagia ostertagi in Irish pasture-based dairy herds. Vet. Parasitol. 2015, 209, 108–116. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Bermúdez, R.; Miranda, M.; Baudracco, J.; Fouz, R.; Pereira, V.; López-Alonso, M. Breeding for organic dairy farming: What types of cows are needed? J. Dairy Res. 2019, 86, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, S.C.; Morris, C.A. Genetics of disease resistance in sheep and goats. Small Rumin. Res. 2007, 70, 48–59. [Google Scholar] [CrossRef]
- Charlier, J.; Duchateau, L.; Claerebout, E.; Williams, D.; Vercruysse, J. Associations between anti-Fasciola hepatica antibody levels in bulk-tank milk samples and production parameters in dairy herds. Prev. Vet. Med. 2007, 78, 57–66. [Google Scholar] [CrossRef] [PubMed]



| 2017 | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|
| % (Pos./Total) | 95% CI | % (Pos./Total) | 95% CI | % (Pos./Total) | 95% CI | |
| O. ostertagia | 36.5 (73/200) | 29.9–43.6% | 41.7 (98/235) | 35.4–48.3% | 45.0 (95/211) | 38.2–52.0% |
| F. hepatica | 15.0 (30/200) | 10.5–20.9% | 11.5 (27/235) | 7.8–16.4% | 18.5 (39/211) | 13.6–24.5% |
| D. viviparus | 2.5 (5/200) | 0.9–6.0% | 1.3 (3/235) | 0.3–4.0% | 3.3 (7/211) | 1.5–7.0% |
| North | East | South | ||||
|---|---|---|---|---|---|---|
| % (pos./total) | 95% CI | % (pos./total) | 95% CI | % (pos./total) | 95% CI | |
| O. ostertagia& F. hepatica | 14.9% (30/201) | 10.4–20.8% | 0.1% (1/204) | 0.0–3.1% | 22.4% (54/241) | 17.4–28.3% |
| O. ostertagia& D. viviparus | 4.5% (9/201) | 2.2–8.6% | 2.5% (5/204) | 0.9–5.9% | 0.4% (1/241) | 0.02–2.7% |
| O. ostertagia& F. hepatica & D. viviparusb | 2.0% (4/201) | 0.6–5.3% | 0.0% (0/204) | 0.0–2.3% | 0.4% (1/241) | 0.02–2.7% |
| North | East | South | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | -2LL | nDF | F | p | OR | 95% CI | -2LL | nDF | F | p | OR | 95% CI | -2LL | nDF | F | p |
| Access to fresh grass | - | - | 185.90 | 1 | 18.47 | <0.001 | - | - | 207.85 | 1 | 11.86 | <0.001 | - | - | 179.54 | 1 | 23.11 | <0.001 |
| Yes vs. no | 5.53 | 2.52–12.12 | - | - | - | - | 3.73 | 1.75–7.94 | - | - | - | - | 6.96 | 3.14–15.42 | - | - | - | - |
| Silage quality | - | - | 185.90 | 2 | 5.47 | 0.005 | - | - | - | - | - | - | - | - | - | - | - | - |
| Lower vs. normal | 0.43 | 0.07–2.54 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Spoiled vs. normal | 3.03 | 1.42–6.48 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Anthelmintic treatment Calves & young cattle | - | - | 185.90 | 2 | 2.33 | 0.100 | - | - | 207.85 | 2 | 1.42 | 0.245 | - | - | 179.54 | 2 | 2.98 | 0.053 |
| Both vs. no treatment | 1.45 | 0.41–5.16 | - | - | - | - | 3.16 | 0.34–29.16 | - | - | - | - | 4.65 | 1.01–21.40 | - | - | - | - |
| Yc vs. no treatment | 2.59 | 1.00–6.71 | - | - | - | - | 1.84 | 0.84–4.02 | - | - | - | - | 2.89 | 1.08–7.78 | - | - | - | - |
| Anthelmintic treatment Lactating & dry cows | - | - | 185.90 | 3 | 1.28 | 0.281 | - | - | 207.85 | 3 | 1.56 | 0.200 | - | - | 179.54 | 2 | 0.26 | 0.771 |
| Both vs. no treatment | 1.00 | 0.44–2.24 | - | - | - | - | 0.26 | 0.06–1.07 | - | - | - | - | 1.12 | 0.33–3.74 | - | - | - | - |
| Lc vs. no treatment | 4.34 | 0.92–20.45 | - | - | - | - | 1.52 | 0.31–7.59 | - | - | - | - | - | - | - | - | - | - |
| Dc vs. no treatment | 0.80 | 0.10–6.16 | - | - | - | - | 1.50 | 0.37–6.01 | - | - | - | - | 2.08 | 0.28–15.27 | - | - | - | - |
| Lameness | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 179.54 | 1 | 1.92 | 0.167 |
| 10% increase from mean | - | - | - | - | - | - | - | - | - | - | - | - | 0.82 | 0.62–1.09 | - | - | - | - |
| Milk yield | - | - | 185.90 | 1 | 6.08 | 0.015 | - | - | 207.85 | 1 | 10.88 | 0.001 | - | - | 179.54 | 1 | 13.08 | <0.001 |
| 500 kg increase from mean | 0.83 | 0.72–0.96 | - | - | - | - | 0.82 | 0.73–0.92 | - | - | - | - | 0.75 | 0.64–0.88 | - | - | - | - |
| North | South | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | -2LL | nDF | F | p | OR | 95% CI | -2LL | nDF | F | p |
| Access to fresh grass | - | - | 125.87 | 1 | 3.50 | 0.063 | - | - | 104.02 | 1 | 15.52 | <0.001 |
| Yes vs. no | 2.74 | 0.95–7.92 | - | - | - | - | 8.71 | 2.95–25.75 | - | - | - | - |
| Hay | - | - | - | - | - | - | - | - | 104.02 | 2 | 4.78 | 0.009 |
| Floor-dried vs. no hay | - | - | - | - | - | - | 12.19 | 1.21–122.50 | - | - | - | - |
| Dried otherwise vs. no hay | - | - | - | - | - | - | 4.97 | 1.46–16.87 | - | - | - | - |
| Anthelmintic treatment Calves & young cattle | - | - | 125.87 | 2 | 1.54 | 0.218 | - | - | 104.02 | 2 | 8.61 | <0.001 |
| Both vs. no treatment | 5.04 | 0.49–52.13 | - | - | - | - | 19.42 | 3.07–122.75 | - | - | - | - |
| Yc vs. no treatment | 6.58 | 0.76–56.76 | - | - | - | - | 16.57 | 4.20–65.43 | - | - | - | - |
| Anthelmintic treatment Lactating & dry cows | - | - | 125.87 | 3 | 0.40 | 0.753 | - | - | 104.02 | 2 | 4.39 | 0.014 |
| Both vs. no treatment | 0.96 | 0.35–2.63 | - | - | - | - | 0.24 | 0.06–1.02 | - | - | - | - |
| Lc vs. no treatment | 1.05 | 0.21–5.34 | - | - | - | - | - | - | - | - | - | - |
| Dc vs. no treatment | 2.62 | 0.41–16.83 | - | - | - | - | 8.37 | 0.94–74.94 | - | - | - | - |
| Lameness | - | - | - | - | - | - | - | - | 104.02 | 1 | 6.69 | 0.010 |
| 10% increase from mean | - | - | - | - | - | - | 0.58 | 0.38–0.88 | - | - | - | - |
| Milk yield | - | - | 125.87 | 1 | - | <0.001 | - | - | 104.02 | 1 | 5.92 | 0.016 |
| 500 kg increase from mean | 0.79 | - | - | - | - | - | 0.75 | 0.60–0.95 | - | - | - | - |
| North (N = 199) a | East (N = 197) | South (N = 230) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | t | p | Est. | SE | t | p | Est. | SE | t | p |
| Intercept | 0.42 | 0.03 | 15.02 | <0.001 | 0.29 | 0.02 | 11.77 | <0.001 | 0.37 | 0.04 | 10.08 | <0.001 |
| O. ostertagi + | 0.02 | 0.03 | 0.88 | 0.382 | −0.02 | 0.02 | −1.18 | 0.241 | 0.03 | 0.02 | 1.13 | 0.261 |
| O. ostertagi ++ | −0.01 | 0.04 | −0.28 | 0.778 | 0.11 | 0.03 | 3.52 | 0.001 | −0.11 | 0.07 | −1.74 | 0.083 |
| F. hepatica + | −1.48 × 10−3 | 0.10 | −0.01 | 0.989 | 0.18 | 0.12 | 1.51 | 0.133 | −0.14 | 0.07 | −2.01 | 0.046 |
| F. hepatica ++ | −0.04 | 0.10 | −0.39 | 0.700 | - | - | - | - | −0.20 | 0.13 | −1.50 | 0.135 |
| F. hepatica +++ | 0.11 | 0.07 | 1.64 | 0.103 | −0.08 | 0.09 | −0.95 | 0.345 | 0.02 | 0.09 | 0.23 | 0.820 |
| O. ostertagi +/F. hepatica + | 0.03 | 0.13 | 0.26 | 0.793 | - | - | - | - | −0.01 | 0.08 | −0.12 | 0.903 |
| O. ostertagi ++/F. hepatica + | 0.18 | 0.13 | 1.33 | 0.187 | - | - | - | - | 0.32 | 0.12 | 2.75 | 0.006 |
| O. ostertagi +/F. hepatica ++ | 0.13 | 0.15 | 0.88 | 0.381 | - | - | - | - | 0.18 | 0.14 | 1.31 | 0.193 |
| O. ostertagi ++/F. hepatica ++ | 0.33 | 0.13 | 2.59 | 0.010 | - | - | - | - | 0.43 | 0.16 | 2.72 | 0.007 |
| O. ostertagi +/F. hepatica +++ | −0.20 | 0.09 | −2.18 | 0.030 | - | - | - | - | −0.05 | 0.10 | −0.55 | 0.580 |
| O. ostertagi ++/F. hepatica +++ | - | - | - | - | - | - | - | - | - | - | - | - |
| D. viviparus + | 0.10 | 0.06 | 1.74 | 0.084 | −0.07 | 0.06 | −1.26 | 0.209 | - | - | - | - |
| Breed type (DP vs. HD) | −0.37 | 0.11 | −3.53 | 0.001 | −0.03 | 0.09 | −0.32 | 0.750 | −0.10 | 0.03 | −3.63 | <0.001 |
| Breed type (Mix vs. HD) | −0.02 | 0.11 | −0.20 | 0.842 | −0.06 | 0.04 | −1.67 | 0.097 | −0.08 | 0.04 | −2.26 | 0.025 |
| No. of scored cows | −5.82 × 10−4 | 2.20 × 10−4 | −2.65 | 0.009 | −4.22 × 10−4 | 9.83 × 10−5 | −4.29 | <0.001 | −1.75 × 10−3 | 3.54 × 10−4 | −4.95 | <0.001 |
| North (N = 192) | East (N = 193) a | South (N = 207) b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | t | p | Est. | SE | t | p | Est. | SE | t | p |
| Intercept | 6441.89 | 1985.87 | 3.24 | 0.001 | −167.19 | 2605.5 | −0.06 | 0.949 | 9611.54 | 1590.34 | 6.0 | <0.001 |
| O. ostertagi + | −153.97 | 196.78 | −0.78 | 0.435 | −195.30 | 193.75 | −1.01 | 0.315 | −219.67 | 188.72 | −1.16 | 0.246 |
| O. ostertagi ++ | −40.11 | 312.66 | −0.13 | 0.898 | −832.74 | 326.22 | −2.55 | 0.012 | −403.61 | 346.48 | −1.17 | 0.246 |
| F. hepatica + | −1157.71 | 372.17 | −3.11 | 0.002 | 1416.02 | 1106.52 | 1.28 | 0.202 | 132.62 | 469.94 | 0.28 | 0.778 |
| F. hepatica ++ | −1335.25 | 445.64 | −3.00 | 0.003 | - | - | - | - | −273.37 | 316.56 | −0.86 | 0.389 |
| F. hepatica +++ | −1129.20 | 376.19 | −3.00 | 0.003 | - | - | - | - | 985.30 | 355.25 | 2.77 | 0.006 |
| D. viviparus + | 159.61 | 430.40 | 0.37 | 0.711 | 724.11 | 509.20 | 1.42 | 0.157 | - | - | - | - |
| Breed type (DP vs. HD) | −2236.55 | 855.00 | −2.62 | 0.010 | −1576.49 | 785.00 | −2.01 | 0.046 | −1259.64 | 293.53 | −4.29 | <0.001 |
| Breed type (Mix vs. HD) | 721.34 | 861.16 | 0.84 | 0.403 | −1015.21 | 357.5 | −2.84 | 0.005 | −1399.50 | 370.78 | −3.77 | <0.001 |
| Lactation no. | 2723.21 | 1330.63 | 2.05 | 0.042 | 7844.89 | 1847.15 | 4.25 | <0.001 | −289.28 | 1037.87 | −0.28 | 0.781 |
| Lactation no. (squared) | −504.38 | 225.54 | −2.24 | 0.027 | −1458.48 | 328.97 | −4.43 | <0.001 | 52.26 | 166.83 | 0.31 | 0.754 |
| SCC (×1000/mL) | −4.27 | 1.06 | −4.01 | <0.001 | −2.63 | 0.90 | −2.93 | 0.004 | −2.77 | 0.85 | −3.26 | 0.001 |
| No. of animals | 3.92 | 1.71 | 2.29 | 0.023 | 0.59 | 0.23 | 2.50 | 0.013 | 7.95 | 2.57 | 3.10 | 0.002 |
| farm type (ORG vs. CON) | −1522.86 | 522.11 | −2.92 | 0.004 | −2446.93 | 343.01 | −7.13 | <0.001 | −1685.37 | 266.41 | −6.33 | <0.001 |
| farm type (TRA vs. CON) | - | - | - | - | - | - | - | - | −245.64 | 399.71 | −0.62 | 0.540 |
| Breed (DP)/F. hepatica + | - | - | - | - | - | - | - | - | 759.10 | 648.05 | 1.17 | 0.243 |
| Breed (Mix)/F. hepatica + | - | - | - | - | - | - | - | - | 498.60 | 868.11 | 0.57 | 0.566 |
| Breed (DP)/F. hepatica ++ | - | - | - | - | - | - | - | - | −2.10 | 692.58 | −0.00 | 0.998 |
| Breed (Mix)/F. hepatica ++ | - | - | - | - | - | - | - | - | - | - | - | - |
| Breed (DP)/F. hepatica +++ | - | - | - | - | - | - | - | - | 1430.93 | 685.62 | 2.09 | 0.038 |
| Breed (Mix)/F. hepatica +++ | - | - | - | - | - | - | - | - | 2050.29 | 1128.19 | 1.82 | 0.071 |
| North (N = 192) | East (N = 195) a | South (N = 208) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | t | p | Est. | SE | t | p | Est. | SE | t | p |
| Intercept | 3.58 | 0.15 | 24.03 | <0.001 | 3.78 | 0.22 | 17.11 | <0.001 | 4.08 | 0.25 | 16.58 | <0.001 |
| O. ostertagi + | −0.01 | 0.01 | −0.87 | 0.388 | 0.01 | 0.02 | 0.43 | 0.666 | 4.36 × 10−3 | 0.02 | 0.21 | 0.831 |
| O. ostertagi ++ | 2.81 × 10−3 | 0.02 | 0.12 | 0.902 | 0.01 | 0.03 | 0.22 | 0.823 | 0.01 | 0.04 | 0.18 | 0.861 |
| F. hepatica + | 0.02 | 0.03 | 0.56 | 0.575 | −0.01 | 0.09 | −0.10 | 0.921 | 0.01 | 0.03 | 0.29 | 0.774 |
| F. hepatica ++ | 0.10 | 0.03 | 3.17 | 0.002 | - | - | - | - | −0.05 | 0.03 | −1.71 | 0.089 |
| F. hepatica +++ | 2.79 × 10−3 | 0.03 | 0.10 | 0.921 | 0.01 | 0.07 | 0.19 | 0.854 | −0.05 | 0.04 | −1.30 | 0.196 |
| D. viviparus + | −2.29 × 10−3 | 0.03 | −0.07 | 0.943 | 0.06 | 0.04 | 1.29 | 0.199 | - | - | - | - |
| Breed type (DP vs. HD) | 0.02 | 0.06 | 0.36 | 0.718 | 3.98 | 1.57 | 2.54 | 0.012 | −0.66 | 0.17 | −3.91 | <0.001 |
| Breed type (Mix vs. HD) | −0.11 | 0.06 | −1.78 | 0.077 | −0.26 | 0.13 | −2.00 | 0.047 | −0.66 | 0.20 | −3.25 | 0.001 |
| Lactation no. | −0.12 | 0.10 | −1.24 | 0.217 | −0.21 | 0.16 | −1.28 | 0.202 | −0.11 | 0.11 | −1.02 | 0.308 |
| Lactation no. (squared) | 0.02 | 0.02 | 1.08 | 0.281 | 0.04 | 0.03 | 1.22 | 0.223 | 0.01 | 0.02 | 0.73 | 0.464 |
| SCC (×1000/mL) | 1.30 × 10−4 | 7.96 × 10−5 | 1.63 | 0.105 | 6.55 × 10−5 | 7.78 × 10−5 | 0.84 | 0.401 | 3.91 × 10−4 | 9.78 × 10−5 | 3.99 | <0.001 |
| Farm type (ORG vs. CON) | −0.10 | 0.04 | −2.45 | 0.015 | −0.10 | 0.03 | −3.15 | 0.002 | −0.10 | 0.03 | −2.99 | 0.003 |
| Farm type (TRA vs. CON) | - | - | - | - | - | - | - | - | −0.08 | 0.04 | −1.75 | 0.082 |
| Milk yield (kg) | - | - | - | - | −1.00 × 10−5 | 6.12 × 10−6 | −1.64 | 0.104 | 4.40 × 10−5 | 1.92 × 10−5 | −2.30 | 0.023 |
| Breed type (DP)/Milk yield | - | - | - | - | −4.52 × 10−4 | 1.86 × 10−4 | −2.43 | 0.016 | 7.52 × 10−5 | 2.02 × 10−5 | 3.72 | <0.001 |
| Breed type (Mix)/Milk yield | - | - | - | - | 3.04 × 10−5 | 1.69 × 10−5 | 1.80 | 0.074 | 7.05 × 10−5 | 2.59 × 10−5 | 2.72 | 0.007 |
| North (N = 192) | East (N = 195) a | South (N = 208) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Est. | SE | t | P | Est. | SE | t | p | Est. | SE | t | p |
| Intercept | 5.36 | 0.31 | 17.07 | <0.001 | 5.26 | 0.45 | 11.67 | <0.001 | 4.60 | 0.43 | 10.67 | <0.001 |
| O. ostertagi + | −0.03 | 0.03 | −1.00 | 0.320 | 0.01 | 0.03 | 0.40 | 0.686 | −0.04 | 0.04 | −1.22 | 0.223 |
| O. ostertagi ++ | −0.04 | 0.05 | −0.88 | 0.378 | 0.03 | 0.06 | 0.61 | 0.545 | −0.13 | 0.06 | −2.01 | 0.046 |
| F. hepatica + | −0.02 | 0.06 | −0.40 | 0.686 | −0.08 | 0.19 | −0.43 | 0.667 | 0.01 | 0.06 | 0.20 | 0.844 |
| F. hepatica ++ | 0.11 | 0.07 | 1.60 | 0.111 | - | - | - | - | −0.09 | 0.06 | −1.68 | 0.094 |
| F. hepatica +++ | 0.05 | 0.06 | 0.85 | 0.394 | −0.11 | 0.14 | −0.83 | 0.406 | −0.12 | 0.06 | −1.84 | 0.067 |
| D. viviparus + | −0.01 | 0.07 | −0.11 | 0.915 | 0.11 | 0.09 | 1.19 | 0.235 | - | - | - | - |
| Breed type (DP vs. HD) | −0.12 | 0.13 | −0.88 | 0.378 | 0.17 | 0.14 | 1.25 | 0.214 | −0.49 | 0.29 | −1.68 | 0.095 |
| Breed type (Mix vs. HD) | −0.15 | 0.13 | −1.16 | 0.249 | 0.01 | 0.06 | 0.15 | 0.885 | −0.83 | 0.35 | −2.35 | 0.020 |
| Lactation no. | −0.34 | 0.21 | −1.62 | 0.106 | −0.35 | 0.33 | −1.05 | 0.294 | 0.20 | 0.19 | 1.04 | 0.301 |
| Lactation no. (squared) | 0.06 | 0.04 | 1.77 | 0.079 | 0.07 | 0.06 | 1.19 | 0.236 | −0.04 | 0.03 | −1.29 | 0.200 |
| SCC (×1000/mL) | −2.12 × 10−4 | 1.71 × 10−4 | −1.24 | 0.216 | −2.29 × 10−4 | 1.59 × 10−4 | −1.45 | 0.150 | 2.36 × 10−4 | 1.68 × 10−4 | 1.41 | 0.161 |
| Herd size | −4.87 × 10−4 | 2.67 × 10−4 | −1.83 | 0.069 | 1.21 × 10−5 | 4.14 × 10−5 | 0.29 | 0.770 | −3.88 × 10−4 | 3.28 × 10−4 | −1.18 | 0.238 |
| farm type (ORG vs. CON) | −0.16 | 0.08 | −1.89 | 0.061 | −0.09 | 0.07 | −1.34 | 0.183 | −0.06 | 0.06 | −1.07 | 0.287 |
| farm type (TRA vs. CON) | - | - | - | - | −0.06 | 0.08 | −0.72 | 0.472 | ||||
| Milk yield (kg) | −7.70 × 10−5 | 1.15 × 10−5 | −6.69 | <0.001 | −8.12 × 10−5 | 1.27 × 10−5 | −6.42 | <0.001 | −7.58 × 10−5 | 3.37 × 10−5 | −2.25 | 0.025 |
| Breed type (DP)/Milk yield | - | - | - | - | - | - | - | - | 5.16 × 10−5 | 3.51 × 10−5 | 1.47 | 0.143 |
| Breed type (Mix)/Milk yield | - | - | - | - | - | - | - | - | 1.04 × 10−4 | 4.50 × 10−5 | 2.31 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; Jordan, D.; Kirse, A.; Schneider, B.; Campe, A.; Knubben-Schweizer, G.; Müller, K.E.; Hoedemaker, M.; Strube, C. Seroprevalence of Major Pasture-Borne Parasitoses (Gastrointestinal Nematodes, Liver Flukes and Lungworms) in German Dairy Cattle Herds, Association with Management Factors and Impact on Production Parameters. Animals 2021, 11, 2078. https://0-doi-org.brum.beds.ac.uk/10.3390/ani11072078
Springer A, Jordan D, Kirse A, Schneider B, Campe A, Knubben-Schweizer G, Müller KE, Hoedemaker M, Strube C. Seroprevalence of Major Pasture-Borne Parasitoses (Gastrointestinal Nematodes, Liver Flukes and Lungworms) in German Dairy Cattle Herds, Association with Management Factors and Impact on Production Parameters. Animals. 2021; 11(7):2078. https://0-doi-org.brum.beds.ac.uk/10.3390/ani11072078
Chicago/Turabian StyleSpringer, Andrea, Daniela Jordan, Alina Kirse, Bettina Schneider, Amely Campe, Gabriela Knubben-Schweizer, Kerstin E. Müller, Martina Hoedemaker, and Christina Strube. 2021. "Seroprevalence of Major Pasture-Borne Parasitoses (Gastrointestinal Nematodes, Liver Flukes and Lungworms) in German Dairy Cattle Herds, Association with Management Factors and Impact on Production Parameters" Animals 11, no. 7: 2078. https://0-doi-org.brum.beds.ac.uk/10.3390/ani11072078