Quercetin Alleviates Endoplasmic Reticulum Stress-Induced Apoptosis in Buffalo Ovarian Granulosa Cells
Abstract
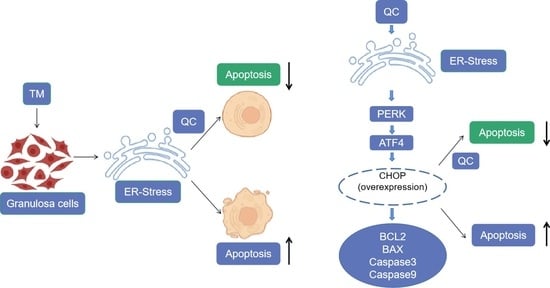
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Quercetin Treatment of GCs
2.3. Cell Immunofluorescence Analysis
2.4. Flow Cytometric Analysis
2.5. ROS Activity Detection
2.6. Quantitative Real-Time PCR
2.7. Western Blot Analysis
2.8. Construction of a Plasmid
2.9. Cell Transfection
2.10. Statistical Analysis
3. Results
3.1. GCs Culture
3.2. Construction of an ER Stress Model
3.3. Effect of QC on TM-Induced Apoptosis in Buffalo GCs
3.4. Effect of QC on Gene Expression in GCs
3.5. Activation of the PERK/CHOP Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shamseddin, M.; De Martino, F.; Constantin, C.; Shamseddin, M.; De Martino, F.; Constantin, C.; Scabia, V.; Lancelot, A.; Laszlo, C.; Ayyannan, A.; et al. Contraceptive progestins with androgenic properties stimulate breast epithelial cell proliferation. Embo Mol. Med. 2021, 13, e14314. [Google Scholar] [CrossRef]
- Smith, M.; Wilkinson, S. ER homeostasis and autophagy. Essays Biochem. 2017, 61, 625–635. [Google Scholar] [PubMed] [Green Version]
- Zhang, H.; Hu, W.; Zhong, Y.; Guo, Z. Meta-analysis of the effects of smooth endoplasmic reticulum aggregation on birth outcome. BMC Pregnancy Childbirth 2021, 21, 374. [Google Scholar] [CrossRef] [PubMed]
- Ogbechi, J.; Hall, B.; Sbarrato, T.; Taunton, J.; Willis, A.E.; Wek, R.C.; Simmonds, R.E. Inhibition of Sec61-dependent translocation by mycolactone uncouples the integrated stress response from ER stress, driving cytotoxicity via translational activation of ATF4. Cell Death Dis. 2018, 9, 397. [Google Scholar] [CrossRef] [Green Version]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic reticulum and the unfolded protein response: Dynamics and metabolic integration. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [PubMed] [Green Version]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ronai, Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Woehlbier, U.; Hetz, C. Modulating stress responses by the UPRosome: A matter of life and death. Trends Biochem. Sci. 2011, 36, 329–337. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Liu, Y. Hydrogen peroxide induces nucleus pulposus cell apoptosis by ATF4/CHOP signaling pathway. Exp. Ther. Med. 2020, 20, 3244–3252. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, L.; Wen, D.; Yang, L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J. Ovarian. Res. 2019, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Rose, B.I.; Brown, S.E. A review of the physiology behind letrozole applications in infertility: Are current protocols optimal? J. Assist. Reprod. Genet. 2020, 37, 2093–2104. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, S.; Gong, F.; Lu, C.; Lin, G.; Hu, L. The mitochondrial DNA copy number of cumulus granulosa cells may be related to the maturity of oocyte cytoplasm. Hum. Reprod. 2020, 35, 1120–1129. [Google Scholar] [CrossRef]
- Dewailly, D.; Lujan, M.E.; Carmina, E.; Cedars, M.I.; Laven, J.; Norman, R.J.; Escobar-Morreale, H.F. Definition and significance of polycystic ovarian morphology: A task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum. Reprod. Update 2014, 20, 334–352. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Chuammitri, P.; Srikok, S.; Saipinta, D.; Boonyayatra, S. The effects of quercetin on microRNA and inflammatory gene expression in lipopolysaccharide-stimulated bovine neutrophils. Vet. World 2017, 10, 403–410. [Google Scholar] [CrossRef]
- Tvrda, E.; Tusimova, E.; Kovacik, A.; Paal, D.; Libova, L.; Lukac, N. Protective Effects of Quercetin on Selected Oxidative Biomarkers in Bovine Spermatozoa Subjected to Ferrous Ascorbate. Reprod. Domest. Anim. 2016, 51, 524–537. [Google Scholar] [CrossRef]
- Sun, T.; Huang, G.; Sun, J.; Wang, Z.; Teng, S.; Cao, Y.; Hanif, Q.; Chen, N.; Lei, C.; Liao, Y. Mitogenome Diversity and Maternal Origins of Guangxi Buffalo Breeds. Animals 2020, 10, 547. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, W.; Wen, A.; Yang, B.; Pang, X. Luzindole and 4P-PDOT block the effect of melatonin on bovine granulosa cell apoptosis and cell cycle depending on its concentration. Peerj 2021, 9, e10627. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Paulmurugan, R.; Ramkumar, K.M. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014, 47, 231–240. [Google Scholar] [CrossRef]
- Reiling, J.H.; Clish, C.B.; Carette, J.E.; Varadarajan, M.; Brummelkamp, T.R.; Sabatini, D.M. A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc. Natl. Acad. Sci. USA 2011, 108, 11756–11765. [Google Scholar] [CrossRef] [Green Version]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-beta-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Şahiner, H.S.; Epikmen, E.T.; Fiaz, M.; Yousuf, M.R.; Khan, S.A.; Serin, I.; Ceylan, A.; Aksoy, M. Dietary quercetin maintains the semen quality in rabbits under summer heat stress. Theriogenology 2018, 122, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, Z.; Aleyasin, A.; Eslami, M.; Nekoonam, S.; Zendedel, A.; Bahramrezaie, M.; Amidi, F. Quercetin protects human granulosa cells against oxidative stress via thioredoxin system. Reprod. Biol. 2019, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Bao, L.; Ding, Y.; Dai, X.; Zhang, Z.; Li, Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017, 15, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Ding, J.; Zhang, A.; Dai, W.; Liu, S.; Diao, Z.; Wang, L.; Han, X.; Liu, W. The Inhibitory Effect of Quercetin on Asymmetric Dimethylarginine-Induced Apoptosis Is Mediated by the Endoplasmic Reticulum Stress Pathway in Glomerular Endothelial Cells. Int. J. Mol. Sci. 2014, 15, 484–503. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Lin, J.; Mi, Y.; Zhang, C. Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol. 2011, 31, 477–485. [Google Scholar] [CrossRef]
- Weng, S.; Mao, L.; Gong, Y.; Sun, T.; Gu, Q. Role of quercetin in protecting ARPE19 cells against H2O2 induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition. Mol. Med. Rep. 2017, 16, 3461–3468. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.S.; Kwon, D.Y.; Kim, M.S.; Kim, H.K.; Lee, Y.C.; Park, S.J.; Yoo, W.H.; Chae, S.; Chung, M.; Kim, H.; et al. The involvement of endoplasmic reticulum stress in flavonoid-induced protection on cardiac cell death caused by ischaemia/reperfusion. J. Pharm. Pharmacol. 2010, 62, 197–204. [Google Scholar] [CrossRef]
- Athanasiou, D.; Aguila, M.; Bellingham, J.; Kanuga, N.; Adamson, P.; Cheetham, M.E. The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Hum. Mol. Genet. 2017, 26, 4896–4905. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Liu, R.; Sun, Q.; Huang, X.; Zhang, J.; Huang, L.; Zhang, P.; Zhang, M.; Fu, Q. Quercetin Alleviates Endoplasmic Reticulum Stress-Induced Apoptosis in Buffalo Ovarian Granulosa Cells. Animals 2022, 12, 787. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12060787
Yang W, Liu R, Sun Q, Huang X, Zhang J, Huang L, Zhang P, Zhang M, Fu Q. Quercetin Alleviates Endoplasmic Reticulum Stress-Induced Apoptosis in Buffalo Ovarian Granulosa Cells. Animals. 2022; 12(6):787. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12060787
Chicago/Turabian StyleYang, Weihan, Runfeng Liu, Qinqiang Sun, Xingchen Huang, Junjun Zhang, Liangfeng Huang, Pengfei Zhang, Ming Zhang, and Qiang Fu. 2022. "Quercetin Alleviates Endoplasmic Reticulum Stress-Induced Apoptosis in Buffalo Ovarian Granulosa Cells" Animals 12, no. 6: 787. https://0-doi-org.brum.beds.ac.uk/10.3390/ani12060787