COVID-19: Test, Test and Test
Abstract
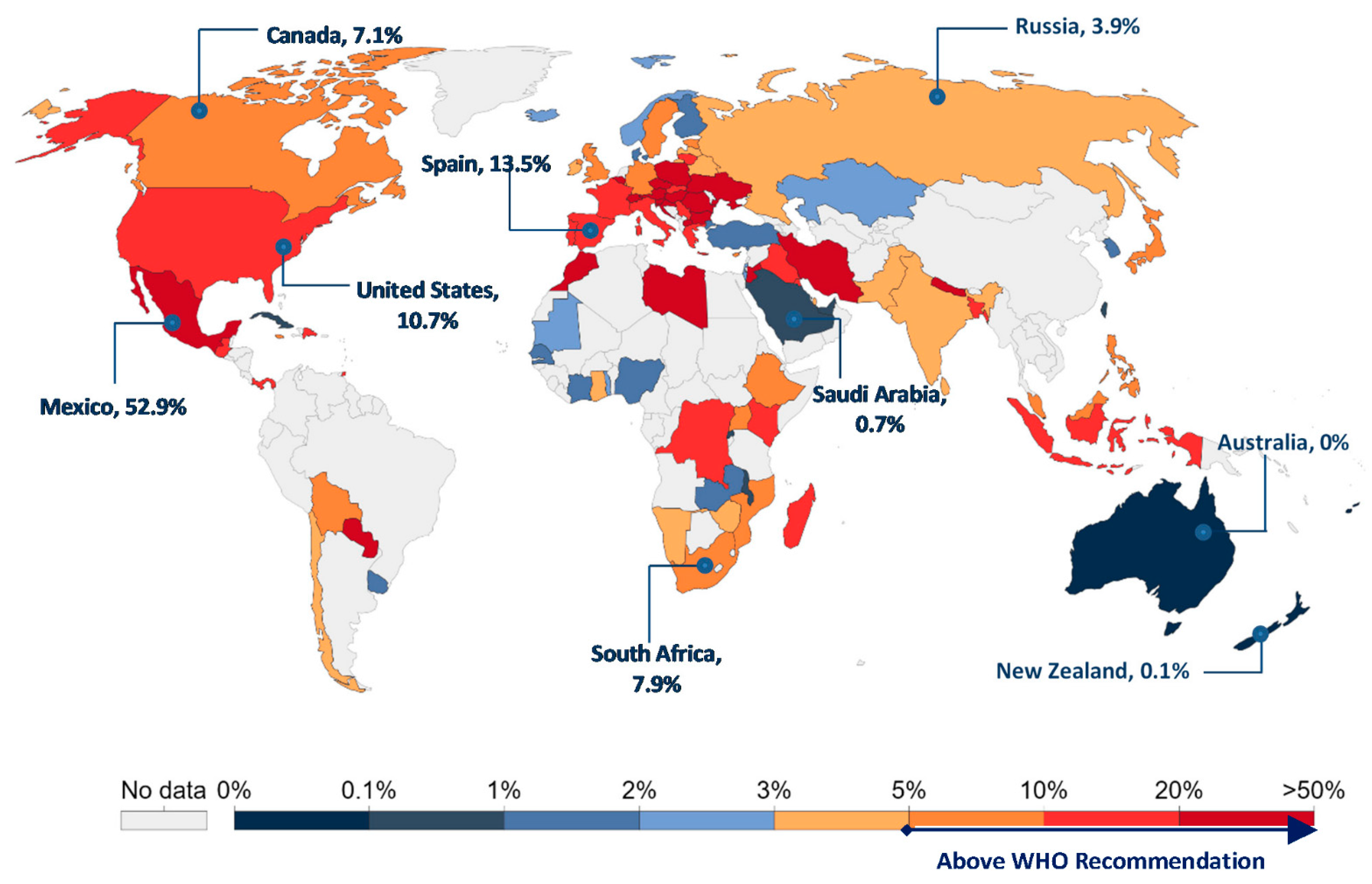
:1. Introduction
2. COVID-19 Diagnostic Testing
3. Sampling Methods
4. Conclusion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus Disease (COVID-19) Dashboard|WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 November 2020).
- Kharroubi, S.; Saleh, F. Are Lockdown Measures Effective Against COVID-19? Front. Public Health 2020, 8, 549692. [Google Scholar] [CrossRef]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 April 2020).
- Kwok, K.O.; Tang, A.; Wei, V.W.I.; Park, W.H.; Yeoh, E.K.; Riley, S. Epidemic Models of Contact Tracing: Systematic Review of Transmission Studies of Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome. Comput. Struct. Biotechnol. J. 2019, 17, 186–194. [Google Scholar] [CrossRef]
- Hellewell, J.; Abbott, S.; Gimma, A.; Bosse, N.I.; Jarvis, C.I.; Russell, T.W.; Munday, J.D.; Kucharski, A.J.; Edmunds, W.J.; Sun, F.; et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health 2020, 8, e488–e496. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus (COVID-19) Testing-Statistics and Research-Our World in Data. Available online: https://ourworldindata.org/coronavirus-testing (accessed on 13 November 2020).
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.F.W.; Yip, C.C.Y.; To, K.K.W.; Tang, T.H.C.; Wong, S.C.Y.; Leung, K.H.; Fung, A.Y.F.; Ng, A.C.K.; Zou, Z.; Tsoi, H.W.; et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J. Clin. Microbiol. 2020, 58, e00310-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colton, H.; Ankcorn, M.; Yavuz, M.; Tovey, L.; Cope, A.; Raza, M.; Keeley, A.J.; State, A.; Poller, B.; Parker, M.; et al. Improved sensitivity using a dual target, E and RdRp assay for the diagnosis of SARS-CoV-2 infection: Experience at a large NHS Foundation Trust in the UK. J. Infect. 2020. [Google Scholar] [CrossRef]
- Peñarrubia, L.; Ruiz, M.; Porco, R.; Rao, S.N.; Juanola-Falgarona, M.; Manissero, D.; López-Fontanals, M.; Pareja, J. Multiple assays in a real-time RT-PCR SARS-CoV-2 panel can mitigate the risk of loss of sensitivity by new genomic variants during the COVID-19 outbreak. Int. J. Infect. Dis. 2020, 97, 225–229. [Google Scholar] [CrossRef]
- Homepage|European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en (accessed on 11 November 2020).
- Xiao, A.T.; Tong, Y.X.; Zhang, S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J. Med. Virol. 2020, 92, 1755–1756. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yao, L.; Li, J.; Chen, L.; Song, Y.; Cai, Z.; Yang, C. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 2020, 92, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.; Wang, X.; Liu, W.; Lu, Y.; Cheng, L.; Sun, Z. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int. J. Infect. Dis. 2020, 94, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Pondaven-Letourmy, S.; Alvin, F.; Boumghit, Y.; Simon, F. How to perform a nasopharyngeal swab in adults and children in the COVID-19 era. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.; Cox, B.; Snowdon, J.; Bakst, J.; Ley, E.; Grajales, P.; Maggiore, J.; Kahn, S. Comparison of abbott id now and abbott m2000 methods for the detection of sars-cov-2 from nasopharyngeal and nasal swabs from symptomatic patients. J. Clin. Microbiol. 2020, 58, e00798-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Péré, H.; Péré, H.; Péré, H.; Podglajen, I.; Podglajen, I.; Wack, M.; Wack, M.; Flamarion, E.; Mirault, T.; Mirault, T.; et al. Nasal swab sampling for SARS-CoV-2: A convenient alternative in times of nasopharyngeal swab shortage. J. Clin. Microbiol. 2020, 58, e00721-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick-Baw, C.; Morgan, K.; Gaffney, D.; Cazares, Y.; Jaworski, K.; Byrd, A.; Molberg, K.; Cavuoti, D. Saliva as an alternate specimen source for detection of sarscov-2 in symptomatic patients using cepheid xpert xpress sars-cov-2. J. Clin. Microbiol. 2020, 58, e01109-20. [Google Scholar] [CrossRef]
- Sapkota, D.; Søland, T.M.; Galtung, H.K.; Sand, L.P.; Giannecchini, S.; To, K.K.W.; Mendes-Correa, M.C.; Giglio, D.; Hasséus, B.; Braz-Silva, P.H. COVID-19 salivary signature: Diagnostic and research opportunities. J. Clin. Pathol. 2020. [CrossRef]
- SoRelle, J.A.; Mahimainathan, L.; McCormick-Baw, C.; Cavuoti, D.; Lee, F.; Thomas, A.; Sarode, R.; Clark, A.E.; Muthukumar, A. Saliva for use with a point of care assay for the rapid diagnosis of COVID-19. Clin. Chim. Acta 2020, 510, 685–686. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Klochendler, A.; Seidel, M.; Sido, T.; Gurel-Gurevich, O.; Yassour, M.; Meshorer, E.; Benedek, G.; Fogel, I.; Oiknine-Djian, E.; et al. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin. Microbiol. Infect. 2020, 26, 1248–1253. [Google Scholar] [CrossRef]
- Yelin, I.; Aharony, N.; Tamar, E.S.; Argoetti, A.; Messer, E.; Berenbaum, D.; Shafran, E.; Kuzli, A.; Gandali, N.; Shkedi, O.; et al. Evaluation of COVID-19 RT-qPCR Test in Multi sample Pools. Clin. Infect. Dis. 2020, 71, 2073–2078. [Google Scholar] [CrossRef]
- Shani-Narkiss, H.; David Gilday, O.; Yayon, N.; Daniel Landau, I. Efficient and Practical Sample Pooling for High-Throughput PCR Diagnosis of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Deka, S.; Kalita, D. Effectiveness of Sample Pooling Strategies for SARS-CoV-2 Mass Screening by RT-PCR: A Scoping Review. J. Lab. Physicians 2020, 12, 212–218. [Google Scholar]
- Böger, B.; Fachi, M.M.; Vilhena, R.O.; Cobre, A.F.; Tonin, F.S.; Pontarolo, R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am. J. Infect. Control 2020, 49, 21–29. [Google Scholar] [CrossRef]
- Chen, C.; Gao, G.; Xu, Y.; Pu, L.; Wang, Q.; Wang, L.; Wang, W.; Song, Y.; Chen, M.; Wang, L.; et al. SARS-CoV-2-Positive Sputum and Feces After Conversion of Pharyngeal Samples in Patients With COVID-19. Ann. Intern. Med. 2020, 172, 832–834. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Rodino, K.G.; Espy, M.J.; Buckwalter, S.P.; Walchak, R.C.; Germer, J.J.; Fernholz, E.; Boerger, A.; Schuetz, A.N.; Yao, J.D.; Binnicker, M.J. Evaluation of Saline, Phosphate-Buffered Saline, and MinimumEssential Medium as Potential Alternatives to Viral Transport Media for SARS-CoV-2 Testing. J. Clin. Microbiol. 2020, 58, e00590-20. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Lou, J.; Bai, Y.; Wang, M. COVID-19 Disease with Positive Fecal and Negative Pharyngeal and Sputum Viral Tests. Am. J. Gastroenterol. 2020, 115, 790. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Deng, Q.; Zhang, G.; Wu, K.; Ni, L.; Yang, Y.; Liu, B.; Wang, W.; Wei, C.; et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020, 92, 833–840. [Google Scholar] [CrossRef] [Green Version]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Nomoto, H.; Ishikane, M.; Katagiri, D.; Kinoshita, N.; Nagashima, M.; Sadamasu, K.; Yoshimura, K.; Ohmagari, N. Cautious handling of urine from moderate to severe COVID-19 patients. Am. J. Infect. Control 2020, 48, 969–971. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA J. Am. Med. Assoc. 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, Q.; Hu, J.; Zhou, M.; Yu, M.; Li, K.; Xu, D.; Xiao, Y.; Yang, J.; Lu, Y.; et al. Nasopharyngeal Swabs Are More Sensitive Than Oropharyngeal Swabs for COVID-19 Diagnosis and Monitoring the SARS-CoV-2 Load. Front. Med. 2020, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Czumbel, L.M.; Kiss, S.; Farkas, N.; Mandel, I.; Hegyi, A.; Nagy, Á.; Lohinai, Z.; Szakács, Z.; Hegyi, P.; Steward, M.C.; et al. Saliva as a Candidate for COVID-19 Diagnostic Testing: A Meta-Analysis. Front. Med. 2020, 7, 465. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Esmaeilzadeh, E.; Li, Y.; Bosch, R.J.; Li, J.Z. SARS-CoV-2 detection in different respiratory sites: A systematic review and meta-analysis. EBioMedicine 2020, 59, 102903. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Carroll, D.; Ussery, E.; Whitham, H.; Elkins, C.A.; Noble-Wang, J.; Rasheed, J.K.; Lu, X.; Lindstrom, S.; Bowen, V.; et al. Performance of Oropharyngeal Swab Testing Compared With Nasopharyngeal Swab Testing for Diagnosis of Coronavirus Disease 2019—United States, January 2020–February 2020. Clin. Infect. Dis. 2020. [CrossRef]
- Kim, H.A.; Hyun, M.; Lee, J.Y.; Park, S.; Ryoo, N.; Kwon, Y.S.; Park, J.S.; Kim, J.Y.; Jeon, J.C.; Peck, K.R. Detection of SARS-CoV-2 in nasal swabs: Comparison with nasopharyngeal swabs. J. Infect. Dev. Ctries. 2020, 14, 1081–1083. [Google Scholar] [CrossRef]
- Griesemer, S.; Van Slyke, G.; Ehrbar, D.; Strle, K.; Yildirim, T.; Centurioni, D.; Walsh, A.; Chang, A.; Waxman, M.; St. George, K. Evaluation of specimen types and saliva stabilization solutions for SARS-CoV-2 testing. medRxiv 2020. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R.; et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 2020, 71, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef]
- Senok, A.; Alsuwaidi, H.; Atrah, Y.; Al Ayedi, O.; Al Zahid, J.; Han, A.; Al Marzooqi, A.; Al Heialy, S.; Altrabulsi, B.; Abdelwareth, L.; et al. Saliva as an alternative specimen for molecular COVID-19 testing in community settings and population-based screening. Infect. Drug Resist. 2020, 13, 3393–3399. [Google Scholar] [CrossRef]
- Liu, R.; Yi, S.; Zhang, J.; Lv, Z.; Zhu, C.; Zhang, Y. Viral Load Dynamics in Sputum and Nasopharyngeal Swab in Patients with COVID-19. J. Dent. Res. 2020, 99, 1239–1244. [Google Scholar] [CrossRef]
| Specimen | Advantages | Disadvantages | Sensitivity a | Specificity | Ref. |
|---|---|---|---|---|---|
| Nasopharyngeal swabs (NPS) | Gold standard | Supervised sample collection, requires specialized medical personnel with PPE, expensive, reflex sneezing/coughing, high risk of viral transmission, patient discomfort | 98% (CI: 89–100%) | 98.1% (CI: 96.5–99.0%) | [15,35,36] |
| Oropharyngeal swabs (OPS) | High sensitivity if performed along with NPS | Supervised sample collection, requires specialized medical personnel with PPE, expensive, highest rate of aerosol transmission, more likely to have nausea and vomit, reflex sneezing/coughing, patient discomfort | 21.1% (CI: 10.5–31.6%) | 97.6% (CI: 93.9–99.5%) | [15,35,37,38] |
| Nasal swabs | Less invasive, less expensive, self-collection, no patient discomfort | Less accurate | 87.1% (CI: 79.57–93.55%) | 100% (CI: 69.2–100%) | [18,39,40,41] |
| Saliva | Self-collection, easy to obtain, cheap, non-invasive, low rate of aerosol transmission, cost-effective, does not require healthcare workers or PPE, no patient discomfort | Relatively less sensitive than NPS | 91% (CI: 80–99%) | 97.6% (CI: 95.5–98.9%) | [19,20,36,42,43] |
| Sputum | Less invasive than NPS, painless | Not all patients can provide it | 97.2% (CI: 90.3–99.7%) | 90.0% (CI: 73.5–97.9%) | [26,37,41,44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, F.A.; Sleem, A. COVID-19: Test, Test and Test. Med. Sci. 2021, 9, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010001
Saleh FA, Sleem A. COVID-19: Test, Test and Test. Medical Sciences. 2021; 9(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010001
Chicago/Turabian StyleSaleh, Fatima A, and Aleen Sleem. 2021. "COVID-19: Test, Test and Test" Medical Sciences 9, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010001





