Epidemiology, Staging, and Management of Multiple Myeloma
Abstract
:1. Introduction
2. Epidemiology
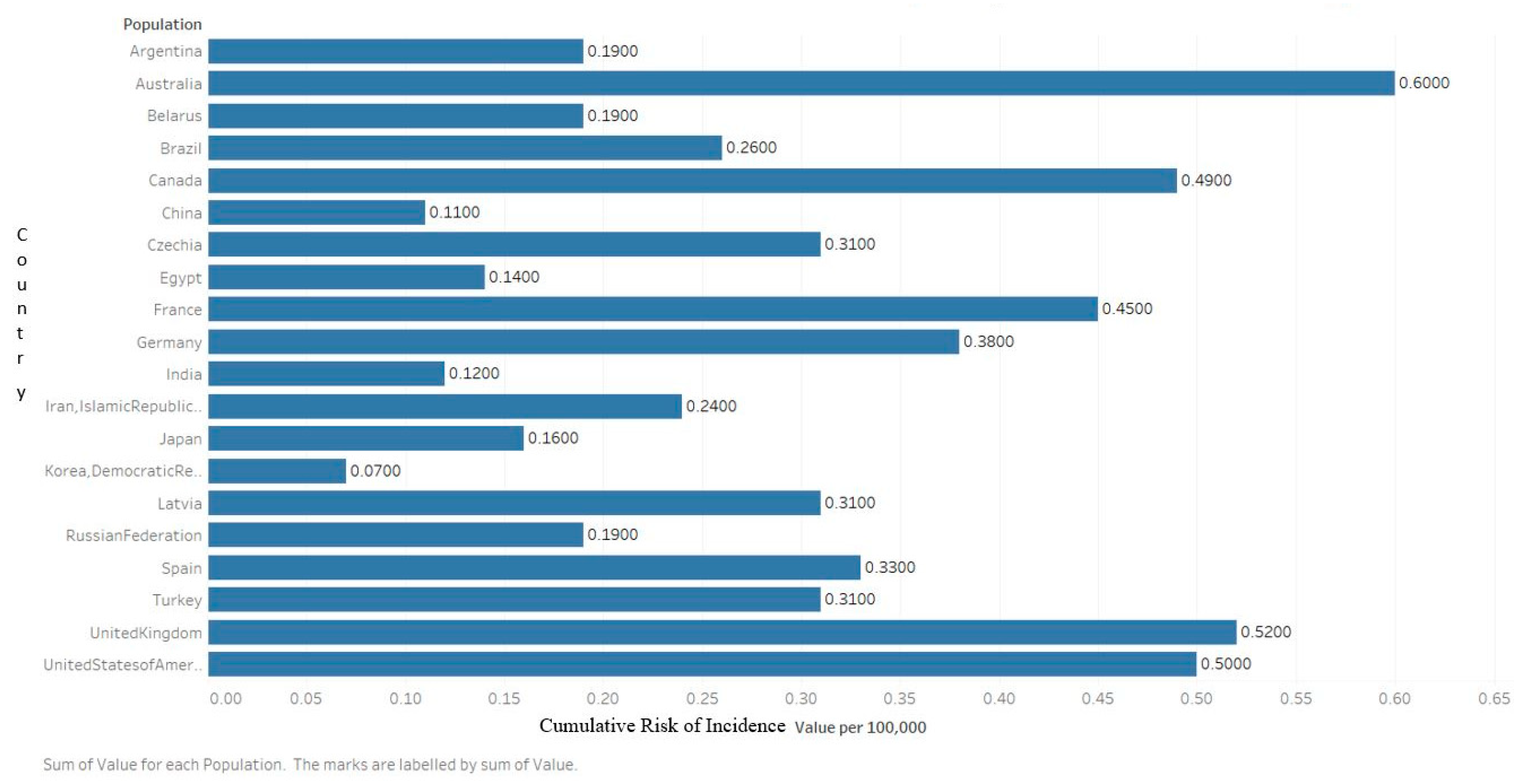
2.1. Incidence
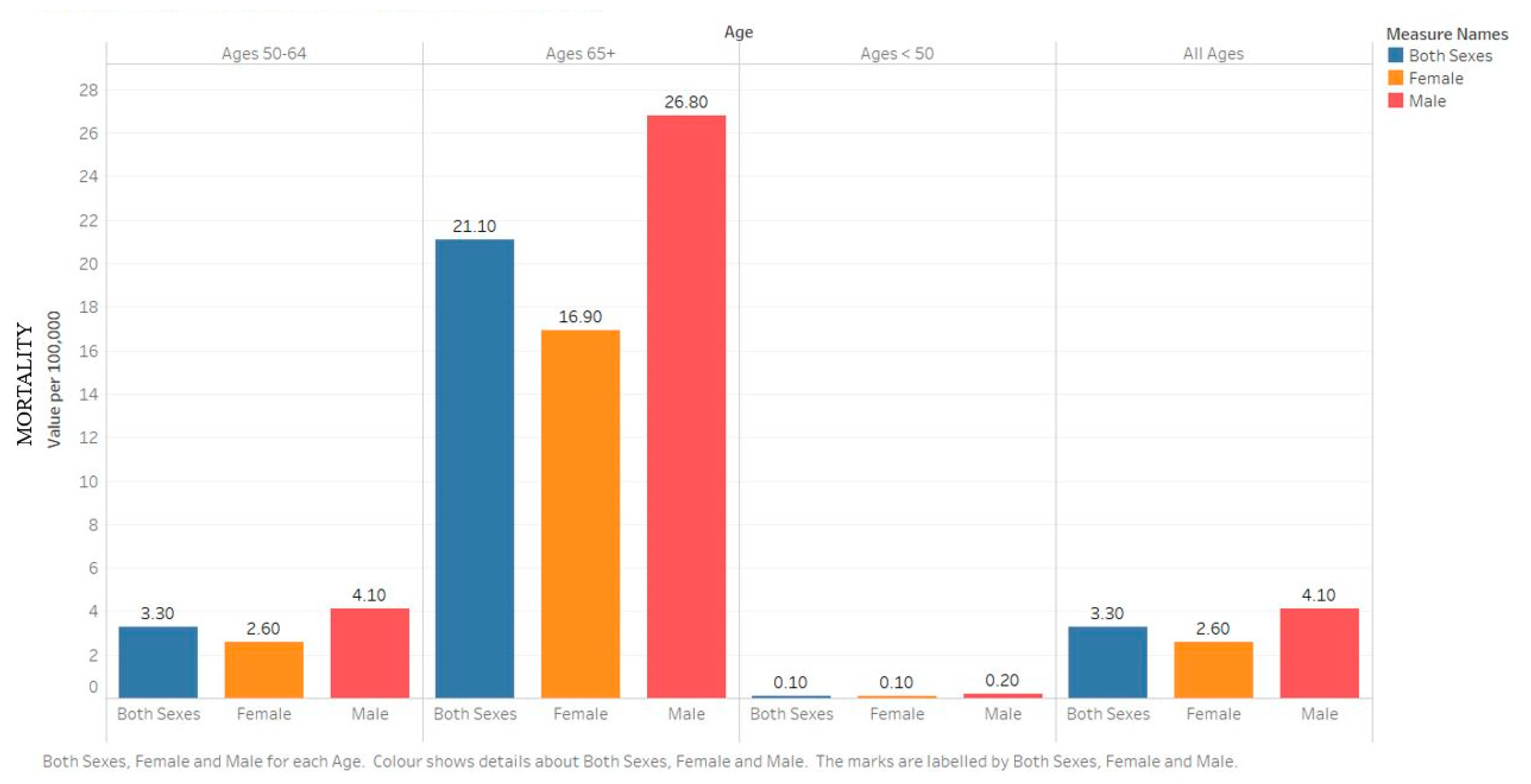
2.2. Mortality
2.3. Risk Factors
2.3.1. Age
2.3.2. Sex
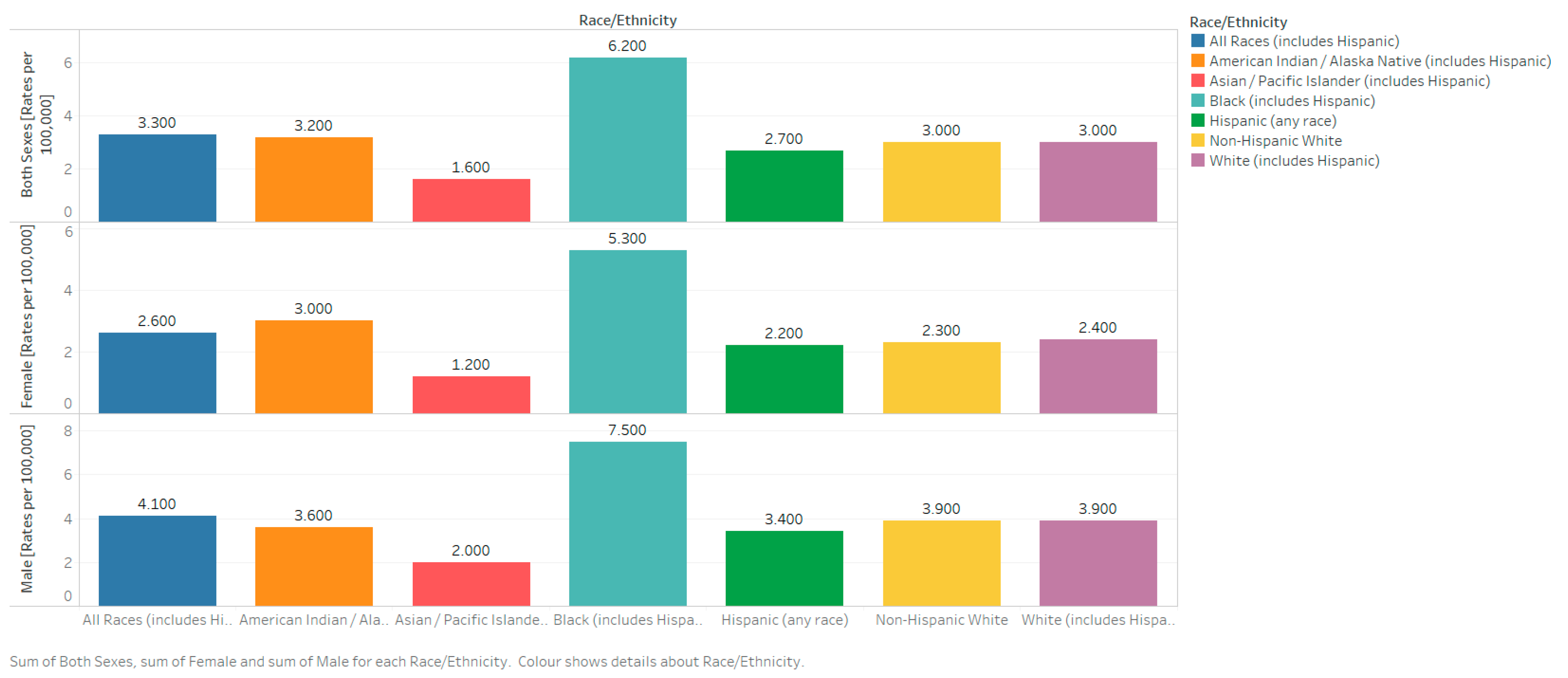
2.3.3. Race
2.3.4. Family History
3. Diagnosis, Staging, and Grading
3.1. Diagnosis
3.2. Staging
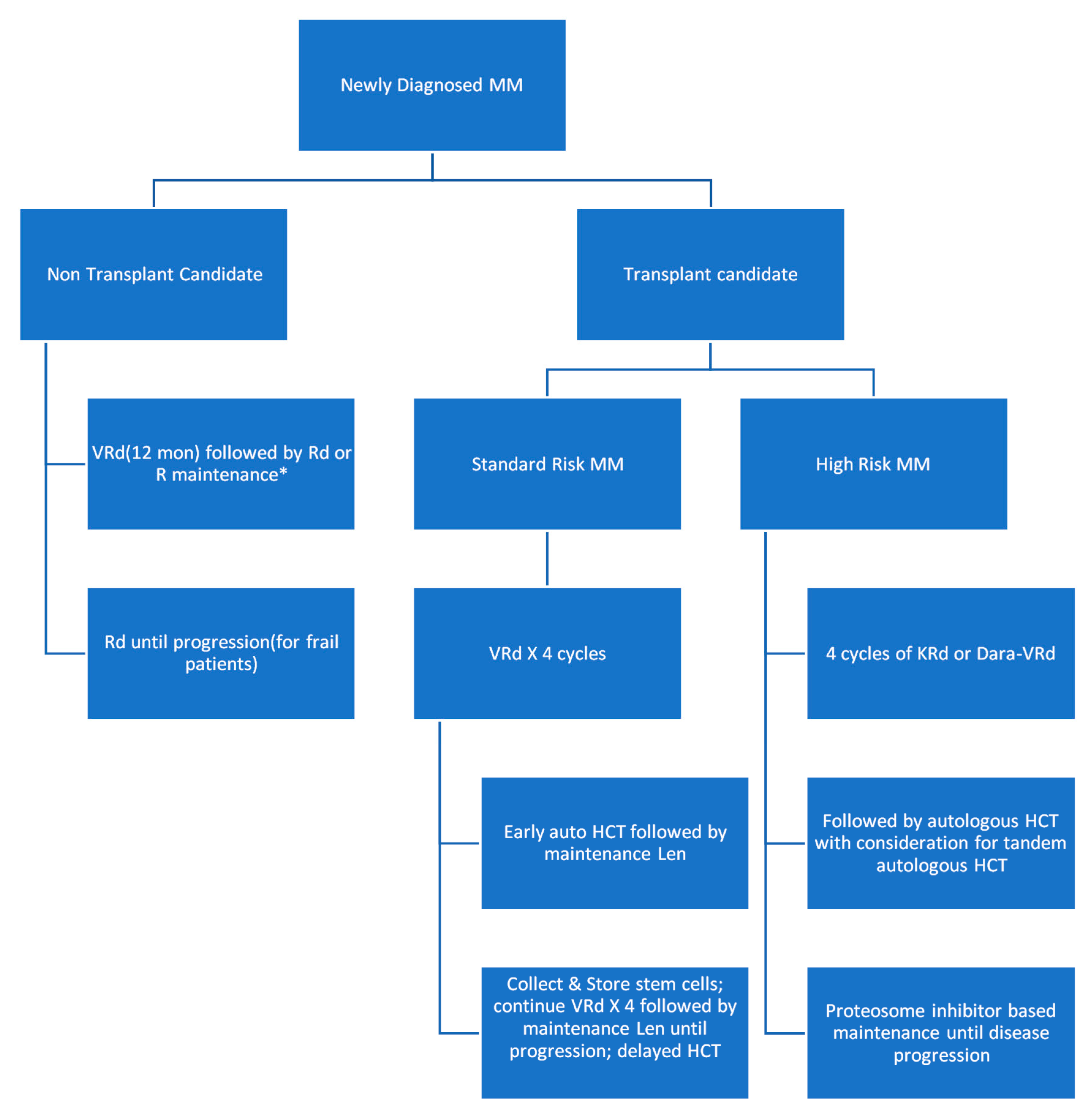
3.3. Management
4. Complications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| SM | smoldering myeloma |
| MGUS | monoclonal gammopathy of undetermined significance |
| CRAB | hypercalcemia, renal failure, anemia, bone involvement |
| ISS | international staging system |
| B2M | beta 2 microglobulin |
| LDH | lactate dehydrogenase |
| HCT | hematopoietic cell transplantation |
| BSAs | bone-stimulating agents |
| GLOBOCAN | Global Cancer Observatory |
| SEER | Surveillance, Epidemiology, and End Results |
| FLCs | Free light chains |
| VRd | bortezomib, lenalidomide, dexamethasone |
| Rd | lenalidomide, dexamethasone |
| VCd also called CyBorD | bortezomib, cyclophosphamide, dexamethasone |
| DRd | daratumumab, lenalidomide, and dexamethasone |
| KRd | carfilzomib, lenalidomide, and dexamethasone |
| RRMM | relapsed/refractory multiple myeloma |
| CNS | central nervous system |
| IMWG | International Myeloma Working Group |
| OS | overall survival |
| PFS | progression-free survival |
| DSS | Durie–Salmon system |
| BCMA-CART | B cell maturation antigen Chimeric antigen receptor T cells |
References
- Jurczyszyn, A.; Suska, A. Multiple Myeloma. Encycl. Biomed. Gerontol. 2019, 2, 461–478. [Google Scholar] [CrossRef]
- Colmone, A.; Amorim, M.; Pontier, A.L.; Wang, S.; Jablonski, E.; Sipkins, D.A. Leukemic Cells Create Bone Marrow Niches That Disrupt the Behavior of Normal Hematopoietic Progenitor Cells. Science 2008, 322, 1861–1865. [Google Scholar] [CrossRef] [Green Version]
- Michels, T.C.; Petersen, K.E. Multiple Myeloma: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 373–383. [Google Scholar]
- Rajkumar, S.V.; Landgren, O.; Mateos, M.-V. Smoldering multiple myeloma. Blood 2015, 125, 3069–3075. [Google Scholar] [CrossRef]
- Mateos, M.; Landgren, O. MGUS and smoldering multiple myeloma: Diagnosis and epidemiology. In Plasma Cell Dyscrasias; Springer: Cham, Switzerland, 2016; Volume 169, pp. 3–12. [Google Scholar] [CrossRef]
- Oza, A.M.; Rajkumar, S.V. Waldenstrom macroglobulinemia: Prognosis and management. Blood Cancer J. 2015, 5, e394. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed on 7 May 2020).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlander, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019.
- SEER*Explorer. Available online: https://seer.cancer.gov/explorer/index.html (accessed on 7 May 2020).
- Atkin, C.; Reddy-Kolanu, V.; Drayson, M.T.; Sapey, E.; Richter, A.G. The prevalence and significance of monoclonal gammopathy of undetermined significance in acute medical admissions. Br. J. Haematol. 2020, 189, 1127–1135. [Google Scholar] [CrossRef]
- Waxman, A.J.; Mink, P.J.; Devesa, S.S.; Anderson, W.F.; Weiss, B.M.; Kristinsson, S.Y.; McGlynn, K.A.; Landgren, O. Racial disparities in incidence and outcome in multiple myeloma: A population-based study. Blood 2010, 116, 5501–5506. [Google Scholar] [CrossRef] [Green Version]
- Weiss, B.M. Multiethnic myeloma. Blood 2013, 121, 3062–3064. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.; Braggio, E.; Jacobus, S.; Jung, S.; Larson, D.; Therneau, T.; Dispenzieri, A.; Van Wier, S.A.; Ahmann, G.; Levy, J.; et al. Uncovering the biology of multiple myeloma among African Americans: A comprehensive genomics approach. Blood 2013, 121, 3147–3152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landgren, O.; Weiss, B.M. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: Support for genetic factors in pathogenesis. Leukemia 2009, 23, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Schinasi, L.H.; Brown, E.E.; Camp, N.J.; Wang, S.S.; Hofmann, J.N.; Chiu, B.C.; Miligi, L.; Freeman, L.E.B.; De Sanjose, S.; Bernstein, L.; et al. Multiple myeloma and family history of lymphohaematopoietic cancers: Results from the International Multiple Myeloma Consortium. Br. J. Haematol. 2016, 175, 87–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Kumar, S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clin. Proc. 2016, 91, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e418–e423. [Google Scholar] [CrossRef]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Hari, P.; Zhang, M.-J.; Roy, V.; Pérez, W.S.; Bashey, A.; To, L.B.; Elfenbein, G.J.; Freytes, C.O.; Gale, R.P.; Gibson, J.; et al. Is the international staging system superior to the Durie–Salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia 2009, 23, 1528–1534. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.G.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Facon, T. Frontline therapy of multiple myeloma. Blood 2015, 125, 3076–3084. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Flinn, I.; Richardson, P.G.; Hari, P.; Callander, N.; Noga, S.J.; Stewart, A.K.; Turturro, F.; Rifkin, R.; Wolf, J.; et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 2012, 119, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Facon, T.; Kumar, S.K.; Plesner, D.T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, M.S.; Nahi, H.; Hulin, C.; Quach, M.H.; et al. Phase 3 Randomized Study of Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Ineligible for Transplant (MAIA). Blood 2018, 132, LBA-2. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2012 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2011, 87, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V. Treatment of multiple myeloma. Nat. Rev. Clin. Oncol. 2011, 8, 479–491. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2010, 86, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Z.; Li, G.; Li, B.; Liu, Y.; Wang, L. Carfilzomib-containing combinations as frontline therapy for multiple myeloma: A meta-analysis of 13 trials. Eur. J. Haematol. 2017, 98, 601–607. [Google Scholar] [CrossRef]
- Miguel, J.F.S.; Schlag, R.; Khuageva, N.K.; Dimopoulos, M.A.; Shpilberg, O.; Kropff, M.; Spicka, I.; Petrucci, M.T.; Palumbo, A.; Samoilova, O.S.; et al. Bortezomib plus Melphalan and Prednisone for Initial Treatment of Multiple Myeloma. N. Engl. J. Med. 2008, 359, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–262. [Google Scholar] [CrossRef]
- Laubach, J.P.; Garderet, L.; Mahindra, A.; Gahrton, G.; Caers, J.; Sezer, O.; Voorhees, P.M.; Leleu, X.; Johnsen, H.E.; Streetly, M.; et al. Management of relapsed multiple myeloma: Recommendations of the International Myeloma Working Group. Leukemia 2016, 30, 1005–1017. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwenhuizen, L.; Biesma, D.H. Central nervous system myelomatosis: Review of the literature. Eur. J. Haematol. 2007, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wirk, B.; Wingard, J.R.; Moreb, J.S. Extramedullary disease in plasma cell myeloma: The iceberg phenomenon. Bone Marrow Transplant. 2012, 48, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirumani, S.H.; Shinagare, A.B.; Jagannathan, J.P.; Krajewski, K.M.; Munshi, N.C.; Ramaiya, N.H. MRI Features of Extramedullary Myeloma. Am. J. Roentgenol. 2014, 202, 803–810. [Google Scholar] [CrossRef]
- Basali, D.; Chakraborty, R.; Rybicki, L.; Rosko, N.; Reed, J.; Karam, M.; Schlueter, K.; Dysert, H.; Kalaycio, M.; Valent, J. Real-world data on safety and efficacy of venetoclax-based regimens in relapsed/refractory t(11;14) multiple myeloma. Br. J. Haematol. 2020, 189, 1136–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, P.A.; Elder, P.T.; Deighan, W.I.; O’Connor, S.J.; Alexander, H.D. Multiple myeloma with central nervous system relapse. Haematologica 2020, 105, 1780–1790. [Google Scholar] [CrossRef]
- Mhaskar, R.; Redzepovic, J.; Wheatley, K.; Clark, O.A.C.; Miladinovic, B.; Glasmacher, A.; Kumar, A.; Djulbegovic, B. Bisphosphonates in multiple myeloma: A network meta-analysis. Cochrane Database Syst. Rev. 2012, CD003188. [Google Scholar] [CrossRef]
- Raje, N.; Roodman, G.D.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Terpos, E.; Kennedy, L.; Sabatelli, L.; Intorcia, M.; Hechmati, G. A cost-effectiveness analysis of denosumab for the prevention of skeletal-related events in patients with multiple myeloma in the United States of America. J. Med. Econ. 2018, 21, 525–536. [Google Scholar] [CrossRef] [Green Version]
- Bringhen, S.; LaRocca, A.; Rossi, D.; Cavalli, M.; Genuardi, M.; Ria, R.; Gentili, S.; Patriarca, F.; Nozzoli, C.; Levi, A.; et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood 2010, 116, 4745–4753. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.; Pylypenko, H.; Grosicki, S.; Karamanesht, I.; Leleu, X.; Grishunina, M.; Rekhtman, G.; Masliak, Z.; Robak, T.; Shubina, A.; et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011, 12, 431–440. [Google Scholar] [CrossRef]
- Arnulf, B.; Pylypenko, H.; Grosicki, S.; Karamanesht, I.; Leleu, X.; Van De Velde, H.; Feng, H.; Cakana, A.; Deraedt, W.; Moreau, P. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica 2012, 97, 1925–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, M.; Salwender, H.; Haenel, M.; Mai, E.K.; Bertsch, U.; Kunz, C.; Hielscher, T.; Blau, I.W.; Scheid, C.; Hose, D.; et al. Subcutaneous versus intravenous bortezomib in two different induction therapies for newly diagnosed multiple myeloma: An interim analysis from the prospective GMMG-MM5 trial. Haematologica 2015, 100, 964–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Jacobus, S.; Callander, N.S.; Fonseca, R.; Vesole, D.H.; E Williams, M.; Abonour, R.; Siegel, D.S.; Katz, M.; Greipp, P.R. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010, 11, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.D.; Garfall, A.L.; Stadtmauer, E.A.; Melenhorst, J.J.; Lacey, S.F.; Lancaster, E.; Vogl, D.T.; Weiss, B.M.; Dengel, K.; Nelson, A.; et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019, 129, 2210–2221. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, R.; Majhail, N.S. Treatment and disease-related complications in multiple myeloma: Implications for survivorship. Am. J. Hematol. 2020, 95, 672–690. [Google Scholar] [CrossRef]
- Vakiti, A.; Padala, S.A.; Mewawalla, P. Myeloma Kidney; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK499952/ (accessed on 7 May 2020).
- Gonsalves, W.I.; Leung, N.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Dingli, D.; Kapoor, P.; Go, R.S.; et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015, 5, e296. [Google Scholar] [CrossRef] [Green Version]
- Vakiti, A.; Mewawalla, P. Malignancy-Related Hypercalcemia; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK482423/ (accessed on 7 May 2020).
- Leleu, X.; Masszi, T.; Bahlis, N.J.; Viterbo, L.; Baker, B.; Gimsing, P.; Maisnar, V.; Samoilova, O.; Rosiñol, L.; Langer, C.; et al. Patient-reported health-related quality of life from the phase III TOURMALINE-MM1 study of ixazomib-lenalidomide-dexamethasone versus placebo-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma. Am. J. Hematol. 2018, 93, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.E.; Smith, W.K.; Levenson, J.L. Reversible Dementia Due to Thalidomide Therapy for Multiple Myeloma. N. Engl. J. Med. 2003, 348, 1821–1822. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Kristinsson, S.Y.; Pfeiffer, R.M.; Björkholm, M.; Goldin, L.R.; Schulman, S.; Blimark, C.; Mellqvist, U.-H.; Wahlin, A.; Turesson, I.; Landgren, O. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: A population-based study. Blood 2010, 115, 4991–4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornell, R.F.; Ky, B.; Weiss, B.M.; Dahm, C.N.; Gupta, D.K.; Du, L.; Carver, J.R.; Cohen, A.D.; Engelhardt, B.G.; Garfall, A.L.; et al. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J. Clin. Oncol. 2019, 37, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Waxman, A.J.; Clasen, S.; Hwang, W.-T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-associated cardiovascular adverse events: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef] [PubMed]



| Disorder | Diagnostic Criteria |
|---|---|
| SMOLDERING MULTIPLE MYELOMA | Two criteria must be met:
|
| MULTIPLE MYELOMA | Two criteria must be met:
|
| IgM MGUS | Three criteria must be met:
|
| LIGHT-CHAIN MGUS | All the following criteria must be met:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010003
Padala SA, Barsouk A, Barsouk A, Rawla P, Vakiti A, Kolhe R, Kota V, Ajebo GH. Epidemiology, Staging, and Management of Multiple Myeloma. Medical Sciences. 2021; 9(1):3. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010003
Chicago/Turabian StylePadala, Sandeep Anand, Adam Barsouk, Alexander Barsouk, Prashanth Rawla, Anusha Vakiti, Ravindra Kolhe, Vamsi Kota, and Germame Hailegiorgis Ajebo. 2021. "Epidemiology, Staging, and Management of Multiple Myeloma" Medical Sciences 9, no. 1: 3. https://0-doi-org.brum.beds.ac.uk/10.3390/medsci9010003





