Effects of Transcranial Electrical Stimulation on Human Auditory Processing and Behavior—A Review
Abstract
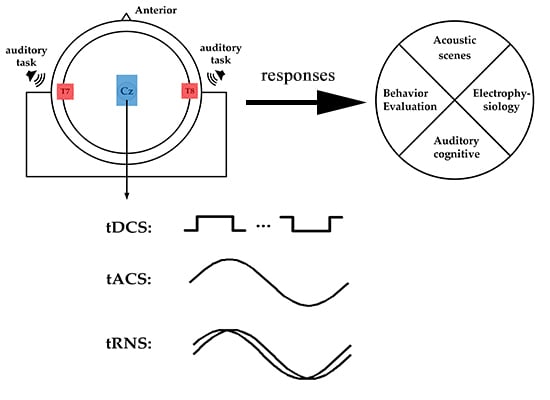
:1. Introduction
2. Physiological Effects of tES on the Auditory Cortex
3. The Potential Processing Effect of tES on the Central Auditory Process
3.1. The Effect of tES on Auditory Temporal Resolution
3.2. The Effect of tES on Auditory Attention
4. The Effect of tES on Speech Processing
4.1. The Effect of tES on Phoneme Classification
4.2. The Effect of tES on the Right Ear Advantage in Speech Processing
4.3. Regulation of tES on Speech Understanding
5. Outline and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| tES | transcranial electrical stimulation |
| tDCS | transcranial direct current stimulation |
| tACS | transcranial alternating current stimulation |
| tRNS | transcranial random noise stimulation |
| HD-tACS | high definition transcranial alternating current stimulation |
| HF-tRNS | high-frequency transcranial random noise stimulation |
| DC | direct current |
| SR | Stochastic resonance |
| ERPs | event-related potentials |
| EEG | electroencephalography |
| AEP | auditory evoked potential |
| SG | sensory gating |
| DLPFC | dorsolateral prefrontal cortex |
| ST | sensory tetanus |
| MMN | mismatch negativity |
| IFG | inferior frontal gyrus |
| AC | auditory cortex |
| ASSR | auditory steady state response |
| MEG | magnetoencephalography |
| ERSP | event-related spectral perturbation |
| ITPC | inter-trial phase coherence |
| LAC | left auditory cortex |
| RAC | right auditory cortex |
| BAC | bilateral auditory cortex |
| RGDT | random gap detection test |
| GDT | gap detection task |
| IGF | individual gamma frequency |
| FEM | finite element model |
| FFT | fast Fourier transform |
| PDT | pitch discrimination task |
| SNR | signal-to-noise ratio |
| STG | superior temporal gyrus |
| pSTG | posterior superior temporal gyrus |
| IPL | inferior parietal lobule |
| SMC | somatosensory-motor cortex |
| PPC | posterior parietal cortex |
| DL | dichotic listening |
| RIPS | right intraparietal sulcus |
| LC-NE | locus coeruleus–norepinephrine |
| VOT | voice onset time |
| CV | consonant-vowels |
| OLSa | Oldenburger sentence test |
| SCT | speech comprehension threshold |
| SRT | sentence reception threshold |
References
- Sporns, O. From simple graphs to the connectome: Networks in neuroimaging. Neuroimage 2012, 62, 881–886. [Google Scholar] [CrossRef]
- Jones, T.A. Motor compensation and its effects on neural reorganization after stroke. Nat. Rev. Neurosci. 2017, 18, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Dubljević, V.; Saigle, V.; Racine, E. The rising tide of tDCS in the media and academic literature. Neuron 2014, 82, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, T.; Cohen Kadosh, R. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Mao, Y.R.; Yuan, T.F.; Xu, D.S.; Cheng, L.M. Multimodal treatment for spinal cord injury: A sword of neuroregeneration upon neuromodulation. Neural Regen. Res. 2020, 15, 1437–1450. [Google Scholar] [PubMed]
- Paulus, W. Transcranial electrical stimulation (tEs—tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.C.; Lomarev, M.; Hallett, M. Modeling the current distribution during transcranial direct current stimulation. Clin. Neurophysiol. 2006, 117, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Neuling, T.; Wagner, S.; Wolters, C.H.; Zaehle, T.; Herrmann, C. Finite-Element Model Predicts Current Density Distribution for Clinical Applications of tDCS and tACS. Front. Psychiatry 2012, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- Bindman, L.J.; Lippold, O.C.; Redfearn, J.W. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents. Nature 1962, 196, 584–585. [Google Scholar] [CrossRef]
- Purpura, D.P.; Scarff, T.; McMurtry, J.G. Intracellular Study of Internuclear Inhibition in Ventrolateral Thalamic Neurons. J. Neurophysiol. 1965, 28, 487–496. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Chaieb, L.; Antal, A.; Walsh, V.; Paulus, W. Frequency-dependent electrical stimulation of the visual cortex. Curr. Biol. 2008, 18, 1839–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiedrzik, C.M. Retina or visual cortex? The site of phosphene induction by transcranial alternating current stimulation. Front. Integr. Neurosci. 2009, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimrath, K.; Fiene, M.; Rufener, K.S.; Zaehle, T. Modulating Human Auditory Processing by Transcranial Electrical Stimulation. Front. Cell. Neurosci. 2016, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Struber, D. Transcranial alternating current stimulation: A review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Moss, F.; Pierson, D.; Ogorman, D. Stochastic Resonance: Tutorial and Update. Int. J. Bifurc. Chaos 1994, 4, 1383–1397. [Google Scholar] [CrossRef]
- Benzi, R.; Sutera, A.; Vulpiani, A. The mechanism of stochastic resonance. J. Phys. A Math. Gen. 1999, 14, L453–L457. [Google Scholar] [CrossRef]
- Moss, F.; Ward, L.M.; Sannita, W.G. Stochastic resonance and sensory information processing: A tutorial and review of application. Clin. Neurophysiol. 2004, 115, 267–281. [Google Scholar] [CrossRef]
- Polania, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Jaberzadeh, S.; Zoghi, M. Non-Invasive Brain Stimulation for Enhancement of Corticospinal Excitability and Motor Performance. Basic Clin. Neurosci. 2013, 4, 257–265. [Google Scholar] [PubMed]
- Antal, A.; Paulus, W.; Nitsche, M.A. Electrical stimulation and visual network plasticity. Restor. Neurol. Neurosci. 2011, 29, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Short, K.; Tanner, D. Event-related potentials (ERPs). In Research Methods in Second Language Psycholinguistics; Routledge: London, UK, 2014; pp. 127–152. [Google Scholar]
- Zaehle, T.; Beretta, M.; Jancke, L.; Herrmann, C.S.; Sandmann, P. Excitability changes induced in the human auditory cortex by transcranial direct current stimulation: Direct electrophysiological evidence. Exp. Brain Res. 2011, 215, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Plourde, G. Auditory evoked potentials. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 129–139. [Google Scholar] [CrossRef]
- Cromwell, H.C.; Mears, R.P.; Wan, L.; Boutros, N.N. Sensory gating: A translational effort from basic to clinical science. Clin. EEG Neurosci. 2008, 39, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, Y.R.; Chen, X.S.; Chen, J.; Wu, Z.G.; Yuan, C.M.; Yi, Z.H.; Hong, W.; Zhang, C.; Cao, L. A follow-up study on features of sensory gating P50 in treatment-resistant depression patients. Chin. Med. J. 2009, 122, 2956–2960. [Google Scholar]
- Wang, Y.; Fu, S.; Greenwood, P.; Luo, Y.; Parasuraman, R. Perceptual load, voluntary attention, and aging: An event-related potential study. Int. J. Psychophysiol. 2012, 84, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Terada, H.; Kurayama, T.; Nakazawa, K.; Matsuzawa, D.; Shimizu, E. Transcranial direct current stimulation (tDCS) onthe dorsolateral prefrontal cortex alters P50 gating. Neurosci. Lett. 2015, 602, 139–144. [Google Scholar] [CrossRef]
- Cieslik, E.C.; Zilles, K.; Caspers, S.; Roski, C.; Kellermann, T.S.; Jakobs, O.; Langner, R.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cereb. Cortex 2013, 23, 2677–2689. [Google Scholar] [CrossRef] [PubMed]
- Rahnev, D.; Nee, D.E.; Riddle, J.; Larson, A.S.; D’Esposito, M. Causal evidence for frontal cortex organization for perceptual decision making. Proc. Natl. Acad. Sci. USA 2016, 113, 6059–6064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philiastides, M.G.; Auksztulewicz, R.; Heekeren, H.R.; Blankenburg, F. Causal role of dorsolateral prefrontal cortex in human perceptual decision making. Curr. Biol. 2011, 21, 980–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzelmann, K.; Meier, L.; Grieder, M.; Morishima, Y.; Dierks, T. No Effect of Transcranial Direct Current Stimulation of the Auditory Cortex on Auditory-Evoked Potentials. Front. Neurosci. 2018, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Picton, T.; Hillyard, S.A.; Krausz, H.I.; Galambos, R. Human auditory evoked potentials. I: Evaluation of components. Electroencephalogr. Clin. Neurophysiol. 1974, 36, 179–190. [Google Scholar] [CrossRef]
- Boroda, E.; Sponheim, S.R.; Fiecas, M.; Lim, K.O. Transcranial direct current stimulation (tDCS) elicits stimulus-specific enhancement of cortical plasticity. Neuroimage 2020, 211, 116598. [Google Scholar] [CrossRef]
- Clapp, W.C.; Hamm, J.P.; Kirk, I.J.; Teyler, T.J. Translating long-term potentiation from animals to humans: A novel method for noninvasive assessment of cortical plasticity. Biol. Psychiatry 2012, 71, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Clapp, W.C.; Kirk, I.J.; Hamm, J.P.; Shepherd, D.; Teyler, T.J. Induction of LTP in the human auditory cortex by sensory stimulation. Eur. J. Neurosci. 2005, 22, 1135–1140. [Google Scholar] [CrossRef]
- Sams, M.; Paavilainen, P.; Alho, K.; Naatanen, R. Auditory frequency discrimination and event-related potentials. Electroencephalogr. Clin. Neurophysiol. 1985, 62, 437–448. [Google Scholar] [CrossRef]
- Chen, J.C.; Hammerer, D.; Strigaro, G.; Liou, L.M.; Tsai, C.H.; Rothwell, J.C.; Edwards, M.J. Domain-specific suppression of auditory mismatch negativity with transcranial direct current stimulation. Clin. Neurophysiol. 2014, 125, 585–592. [Google Scholar] [CrossRef]
- Impey, D.; Knott, V. Effect of transcranial direct current stimulation (tDCS) on MMN-indexed auditory discrimination: A pilot study. J. Neural Transm. 2015, 122, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Impey, D.; de la Salle, S.; Baddeley, A.; Knott, V. Effects of an NMDA antagonist on the auditory mismatch negativity response to transcranial direct current stimulation. J. Psychopharmacol. 2017, 31, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Weigl, M.; Mecklinger, A.; Rosburg, T. Transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates auditory mismatch negativity. Clin. Neurophysiol. 2016, 127, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Kipp, K.H.; Mecklinger, A.; Becker, M.; Reith, W.; Gortner, L. Infant febrile seizures: Changes in declarative memory as revealed by event-related potentials. Clin. Neurophysiol. 2010, 121, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Royal, I.; Zendel, B.R.; Desjardins, M.E.; Robitaille, N.; Peretz, I. Modulation of electric brain responses evoked by pitch deviants through transcranial direct current stimulation. Neuropsychologia 2018, 109, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, P.; Jolicœur, P.; Peretz, I. Pitch discrimination without awareness in congenital amusia: Evidence from event-related potentials. Brain Cogn. 2013, 81, 337–344. [Google Scholar] [CrossRef]
- Brenner, C.A.; Krishnan, G.P.; Vohs, J.L.; Ahn, W.-Y.; Hetrick, W.P.; Morzorati, S.L.; O’Donnell, B.F. Steady State Responses: Electrophysiological Assessment of Sensory Function in Schizophrenia. Schizophr. Bull. 2009, 35, 1065–1077. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.K.; Fries, P.; Singer, W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001, 2, 704–716. [Google Scholar] [CrossRef]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [Green Version]
- Galambos, R.; Makeig, S.; Talmachoff, P.J. A 40-Hz auditory potential recorded from the human scalp. Proc. Natl. Acad. Sci. USA 1981, 78, 2643–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Doren, J.; Langguth, B.; Schecklmann, M. Electroencephalographic effects of transcranial random noise stimulation in the auditory cortex. Brain Stimul. 2014, 7, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Miyagishi, Y.; Ikeda, T.; Takahashi, T.; Kudo, K.; Morise, H.; Minabe, Y.; Kikuchi, M. Gamma-band auditory steady-state response after frontal tDCS: A double-blind, randomized, crossover study. PLoS ONE 2018, 13, e0193422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrino, G.; Arcara, G.; Di Pino, G.; Turco, C.; Maran, M.; Weis, L.; Piccione, F.; Siebner, H.R. Transcranial direct current stimulation over the sensory-motor regions inhibits gamma synchrony. Hum. Brain Mapp. 2019, 40, 2736–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, D.B.; Fried, P.J.; Ruffini, G.; Ripolles, O.; Salvador, R.; Banus, J.; Ketchabaw, W.T.; Santarnecchi, E.; Pascual-Leone, A.; Fox, M.D. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage 2017, 157, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Hyvarinen, P.; Choi, D.; Demarchi, G.; Aarnisalo, A.A.; Weisz, N. tACS-mediated modulation of the auditory steady-state response as seen with MEG. Hear. Res. 2018, 364, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [Green Version]
- Catts, H.W.; Chermak, G.D.; Craig, C.H.; Johnston, J.R.; Keith, R.W.; Musiek, F.E.; Robin, D.A.; Sloan, C.; Paulbrown, D.; Thompson, M.E. Central Auditory Processing: Current Status of Research and Implications for Clinical Practice Task Force on Central Auditory Processing Consensus Development. Am. J. Audiol. 1996, 5, 41–52. [Google Scholar]
- Schönwiesner, M.; Zatorre, R.J. Spectro-temporal modulation transfer function of single voxels in the human auditory cortex measured with high-resolution fMRI. Proc. Natl. Acad. Sci. USA 2009, 106, 14611–14616. [Google Scholar] [CrossRef] [Green Version]
- Ladeira, A.; Fregni, F.; Campanha, C.; Valasek, C.A.; De Ridder, D.; Brunoni, A.; Boggio, P.S. Polarity-dependent transcranial direct current stimulation effects on central auditory processing. PLoS ONE 2011, 6, e25399. [Google Scholar] [CrossRef]
- Heimrath, K.; Kuehne, M.; Heinze, H.; Zaehle, T. Transcranial direct current stimulation (tDCS) traces the predominance of the left auditory cortex for processing of rapidly changing acoustic information. Neuroscience 2014, 261, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Baltus, A.; Herrmann, C.S. Auditory temporal resolution is linked to resonance frequency of the auditory cortex. Int. J. Psychophysiol. 2015, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.; Alain, C.; Schneider, B.A. Within- and between-channel gap detection in the human auditory cortex. Neuroreport 2004, 15, 2051–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baltus, A.; Wagner, S.; Wolters, C.H.; Herrmann, C.S. Optimized auditory transcranial alternating current stimulation improves individual auditory temporal resolution. Brain Stimul. 2018, 11, 118–124. [Google Scholar] [CrossRef]
- Baltus, A.; Vosskuhl, J.; Boetzel, C.; Herrmann, C.S. Transcranial alternating current stimulation modulates auditory temporal resolution in elderly people. Eur. J. Neurosci. 2020, 51, 1328–1338. [Google Scholar] [CrossRef]
- Rufener, K.S.; Ruhnau, P.; Heinze, H.J.; Zaehle, T. Transcranial Random Noise Stimulation (tRNS) Shapes the Processing of Rapidly Changing Auditory Information. Front. Cell. Neurosci. 2017, 11, 162. [Google Scholar] [CrossRef] [Green Version]
- Rufener, K.; Keute, M.; Kauk, J.; Heinze, H.J.; Zaehle, T. P 102 Domain-specific effects of transcranial random noise stimulation (tRNS) on auditory feature processing. Clin. Neurophysiol. 2017, 128, e379. [Google Scholar] [CrossRef]
- Cherry, E.C. Some Experiments on the Recognition of Speech, with One and with Two Ears. Acoust. Soc. Am. J. 1953, 25, 975–979. [Google Scholar] [CrossRef]
- Lewald, J. Modulation of human auditory spatial scene analysis by transcranial direct current stimulation. Neuropsychologia 2016, 84, 282–293. [Google Scholar] [CrossRef]
- Lewald, J. Bihemispheric anodal transcranial direct-current stimulation over temporal cortex enhances auditory selective spatial attention. Exp. Brain Res. 2019, 237, 1539–1549. [Google Scholar] [CrossRef]
- Hanenberg, C.; Getzmann, S.; Lewald, J. Transcranial direct current stimulation of posterior temporal cortex modulates electrophysiological correlates of auditory selective spatial attention in posterior parietal cortex. Neuropsychologia 2019, 131, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lewald, J.; Getzmann, S. Electrophysiological correlates of cocktail-party listening. Behav. Brain Res. 2015, 292, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kerlin, J.R.; Shahin, A.J.; Miller, L.M. Attentional Gain Control of Ongoing Cortical Speech Representations in a “Cocktail Party”. J. Neurosci. 2010, 30, 620–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haegens, S.; Haendel, B.F.; Jensen, O. Top-Down Controlled Alpha Band Activity in Somatosensory Areas Determines Behavioral Performance in a Discrimination Task. J. Neurosci. 2011, 31, 5197–5204. [Google Scholar] [CrossRef]
- Worden, M.S.; Foxe, J.J.; Wang, N.; Simpson, G.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J. Neurosci. 2000, 20, RC63. [Google Scholar] [CrossRef] [Green Version]
- Tzvetan, P.; Sabine, K.; Ole, J. FEF-Controlled Alpha Delay Activity Precedes Stimulus-Induced Gamma-Band Activity in Visual Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 4117–4127. [Google Scholar]
- Wostmann, M.; Vosskuhl, J.; Obleser, J.; Herrmann, C.S. Opposite effects of lateralised transcranial alpha versus gamma stimulation on auditory spatial attention. Brain Stimul. 2018, 11, 752–758. [Google Scholar] [CrossRef]
- Deng, Y.; Reinhart, R.M.; Choi, I.; Shinn-Cunningham, B.G. Causal links between parietal alpha activity and spatial auditory attention. eLife 2019, 8, e51184. [Google Scholar] [CrossRef]
- Golbarg, M.; Barbara, S.-C.; Torsten, D. Influence of talker discontinuity on cortical dynamics of auditory spatial attention. NeuroImage 2018, 179, 548–556. [Google Scholar]
- Nieuwenhuis, S.; Astonjones, G.; Cohen, J.D. Decision making, the P3, and the locus coeruleus--norepinephrine system. Psychol. Bull. 2005, 131, 510–532. [Google Scholar] [CrossRef]
- Rufener, K.S.; Geyer, U.; Janitzky, K.; Heinze, H.J.; Zaehle, T. Modulating auditory selective attention by non-invasive brain stimulation: Differential effects of transcutaneous vagal nerve stimulation and transcranial random noise stimulation. Eur. J. Neurosci. 2018, 48, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Lisker, L.; Abramson, A.S. A Cross-Language Study of Voicing in Initial Stops: Acoustical Measurements. Word 2015, 20, 384–422. [Google Scholar] [CrossRef] [Green Version]
- Heimrath, K.; Fischer, A.; Heinze, H.J.; Zaehle, T. Changed categorical perception of consonant-vowel syllables induced by transcranial direct current stimulation (tDCS). BMC Neurosci. 2016, 17, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Poeppel, D. Cortical oscillations in auditory perception and speech: Evidence for two temporal windows in human auditory cortex. Front. Psychol. 2012, 3, 170. [Google Scholar] [CrossRef] [Green Version]
- Pena, M.; Melloni, L. Brain oscillations during spoken sentence processing. J. Cogn. Neurosci. 2012, 24, 1149–1164. [Google Scholar] [CrossRef]
- Rufener, K.S.; Zaehle, T.; Oechslin, M.S.; Meyer, M. 40Hz-Transcranial alternating current stimulation (tACS) selectively modulates speech perception. Int. J. Psychophysiol. 2016, 101, 18–24. [Google Scholar] [CrossRef]
- Strueber, D.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Antiphasic 40 Hz Oscillatory Current Stimulation Affects Bistable Motion Perception. Brain Topogr. 2014, 27, 158–171. [Google Scholar] [CrossRef]
- Rufener, K.S.; Oechslin, M.S.; Zaehle, T.; Meyer, M. Transcranial Alternating Current Stimulation (tACS) differentially modulates speech perception in young and older adults. Brain Stimul. 2016, 9, 560–565. [Google Scholar] [CrossRef]
- Westerhausen, R.; Hugdahl, K. The corpus callosum in dichotic listening studies of hemispheric asymmetry: A review of clinical and experimental evidence. Neurosci. Biobehav. Rev. 2008, 32, 1044–1054. [Google Scholar] [CrossRef]
- Hugdahl, K. Fifty years of dichotic listening research—Still going and going and …. Brain Cogn. 2011, 76, 211–213. [Google Scholar] [CrossRef]
- D’Anselmo, A.; Prete, G.; Tommasi, L.; Brancucci, A. The Dichotic Right Ear Advantage Does not Change with Transcranial Direct Current Stimulation (tDCS). Brain Stimul. 2015, 8, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, S.; Leicht, G.; Ertl, M.; Andreou, C.; Polomac, N.; Westerhausen, R.; Friederici, A.D.; Mulert, C. Conscious auditory perception related to long-range synchrony of gamma oscillations. Neuroimage 2014, 100, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Nolte, G.; Schneider, T.R.; Engel, A.K.; Leicht, G.; Mulert, C. Intrinsic 40Hz-phase asymmetries predict tACS effects during conscious auditory perception. PLoS ONE 2019, 14, e0213996. [Google Scholar] [CrossRef] [PubMed]
- Della Penna, S.; Brancucci, A.; Babiloni, C.; Franciotti, R.; Pizzella, V.; Rossi, D.; Torquati, K.; Rossini, P.M.; Romani, G.L. Lateralization of dichotic speech stimuli is based on specific auditory pathway interactions: Neuromagnetic evidence. Cereb. Cortex 2007, 17, 2303–2311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanneste, S.; Fregni, F.; De Ridder, D. Head-to-Head Comparison of Transcranial Random Noise Stimulation, Transcranial AC Stimulation, and Transcranial DC Stimulation for Tinnitus. Front. Psychiatry 2013, 4, 158. [Google Scholar] [CrossRef] [Green Version]
- Prete, G.; Danselmo, A.; Tommasi, L.; Brancucci, A. Modulation of the dichotic right ear advantage during bilateral but not unilateral transcranial random noise stimulation. Brain Cogn. 2018, 123, 81–88. [Google Scholar] [CrossRef]
- Peelle, J.E.; Davis, M.H. Neural oscillations carry speech rhythm through to comprehension. Front. Psychol. 2012, 3, 320. [Google Scholar] [CrossRef] [Green Version]
- Ahissar, E.; Nagarajan, S.S.; Ahissar, M.; Protopapas, A.; Mahncke, H.W.; Merzenich, M.M. Speech comprehension is correlated with temporal response patterns recorded from auditory cortex. Proc. Natl. Acad. Sci. USA 2001, 98, 13367–13372. [Google Scholar] [CrossRef] [Green Version]
- Neuling, T.; Rach, S.; Wagner, S.; Wolters, C.H.; Herrmann, C.S. Good vibrations: Oscillatory phase shapes perception. Neuroimage 2012, 63, 771–778. [Google Scholar] [CrossRef]
- Riecke, L.; Formisano, E.; Herrmann, C.S.; Sack, A.T. 4-Hz Transcranial Alternating Current Stimulation Phase Modulates Hearing. Brain Stimul. 2015, 8, 777–783. [Google Scholar] [CrossRef]
- Wilsch, A.; Neuling, T.; Obleser, J.; Herrmann, C.S. Transcranial alternating current stimulation with speech envelopes modulates speech comprehension. Neuroimage 2018, 172, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Zoefel, B.; Allard, I.; Anil, M.; Davis, M.H. Perception of Rhythmic Speech Is Modulated by Focal Bilateral Transcranial Alternating Current Stimulation. J. Cogn. Neurosci. 2020, 32, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Riecke, L.; Sack, A.T.; Schroeder, C.E. Endogenous Delta/Theta Sound-Brain Phase Entrainment Accelerates the Buildup of Auditory Streaming. Curr. Biol. 2015, 25, 3196–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadir, S.; Kaza, C.; Weissbart, H.; Reichenbach, T. Modulation of Speech-in-Noise Comprehension through Transcranial Current Stimulation With the Phase-Shifted Speech Envelope. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, M.; Kegler, M.; Kadir, S.; Reichenbach, T. Transcranial alternating current stimulation in the theta band but not in the delta band modulates the comprehension of naturalistic speech in noise. Neuroimage 2020, 210, 116557. [Google Scholar] [CrossRef]
- Dunn, W.; Rassovsky, Y.; Wynn, J.K.; Wu, A.D.; Iacoboni, M.; Hellemann, G.; Green, M.F. Modulation of neurophysiological auditory processing measures by bilateral transcranial direct current stimulation in schizophrenia. Schizophr. Res. 2016, 174, 189–191. [Google Scholar] [CrossRef]
- Dunn, W.; Rassovsky, Y.; Wynn, J.; Wu, A.D.; Iacoboni, M.; Hellemann, G.; Green, M.F. The effect of bilateral transcranial direct current stimulation on early auditory processing in schizophrenia: A preliminary study. J. Neural Transm. 2017, 124, 1145–1149. [Google Scholar] [CrossRef]
- Dondé, C.; Amad, A.; Nieto, I.; Brunoni, A.R.; Neufeld, N.H.; Bellivier, F.; Poulet, E.; Geoffroy, P.A. Transcranial direct-current stimulation (tDCS) for bipolar depression: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 123–131. [Google Scholar] [CrossRef]
- MF, A.L.; Armijo-Olivo, S.; Kim, E.S. Transcranial direct current stimulation (tDCS) to improve naming ability in post-stroke aphasia: A critical review. Behav. Brain Res. 2017, 332, 7–15. [Google Scholar]
- Vanneste, S.; Pouthas, V.; Wearden, J.H. Temporal control of rhythmic performance: A comparison between young and old adults. Exp. Aging Res. 2001, 27, 83–102. [Google Scholar] [CrossRef]
- Mendelson, J.R.; Ricketts, C. Age-related temporal processing speed deterioration in auditory cortex. Hear. Res. 2001, 158, 84–94. [Google Scholar] [CrossRef]
- Vanneste, S.; De Ridder, D. Bifrontal transcranial direct current stimulation modulates tinnitus intensity and tinnitus-distress-related brain activity. Eur. J. Neurosci. 2011, 34, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Luft, C.D.; Pereda, E.; Banissy, M.J.; Bhattacharya, J. Best of both worlds: Promise of combining brain stimulation and brain connectome. Front. Syst. Neurosci. 2014, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaab, N.; Gabrieli, J.D.; Deutsch, G.K.; Tallal, P.; Temple, E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restor. Neurol. Neurosci. 2007, 25, 295–310. [Google Scholar]
- Madaus, J.W. Employment self-disclosure rates and rationales of university graduates with learning disabilities. J. Learn. Disabil. 2008, 41, 291–299. [Google Scholar] [CrossRef]
- Fertonani, A.; Rosini, S.; Cotelli, M.; Rossini, P.M.; Miniussi, C. Naming facilitation induced by transcranial direct current stimulation. Behav. Brain Res. 2010, 208, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Iyer, M.B.; Mattu, U.; Grafman, J.; Lomarev, M.; Sato, S.; Wassermann, E.M. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 2005, 64, 872–875. [Google Scholar] [CrossRef]
- Rufener, K.S.; Krauel, K.; Meyer, M.; Heinze, H.J.; Zaehle, T. Transcranial electrical stimulation improves phoneme processing in developmental dyslexia. Brain Stimul. 2019, 12, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.I.; Bikson, M.; Datta, A.; Minhas, P.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimul. 2013, 6, 644–648. [Google Scholar] [CrossRef]
- Ali, M.M.; Sellers, K.K.; Fröhlich, F. Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 2013, 33, 11262–11275. [Google Scholar] [CrossRef]
- Ozen, S.; Sirota, A.; Belluscio, M.A.; Anastassiou, C.A.; Stark, E.; Koch, C.; Buzsáki, G. Transcranial electric stimulation entrains cortical neuronal populations in rats. J. Neurosci. 2010, 30, 11476–11485. [Google Scholar] [CrossRef] [PubMed]
- Noury, N.; Siegel, M. Phase properties of transcranial electrical stimulation artifacts in electrophysiological recordings. Neuroimage 2017, 158, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Noury, N.; Hipp, J.F.; Siegel, M. Physiological processes non-linearly affect electrophysiological recordings during transcranial electric stimulation. Neuroimage 2016, 140, 99–109. [Google Scholar] [CrossRef]
- Vanneste, S.; Walsh, V.; Van De Heyning, P.; De Ridder, D. Comparing immediate transient tinnitus suppression using tACS and tDCS: A placebo-controlled study. Exp. Brain Res. 2013, 226, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Stamberger, H.; Van de Heyning, P.; De Ridder, D.; Vanneste, S. Auditory cortex tACS and tRNS for tinnitus: Single versus multiple sessions. Neural Plast. 2014, 2014, 436713. [Google Scholar] [CrossRef] [Green Version]

| Reference | Sample Size | Stimulation Types | Stimulation Electrode Position and Size | Reference Electrode Position and Size | Stimulation Parameters | The Number of Stimulation Sessions | EEG Recording Time | Results |
|---|---|---|---|---|---|---|---|---|
| Zaehle, et al., 2011 [25] | 14 | tDCS: anodal/cathodal/sham | T7 or CP5, 35 cm2 | Contralateral supraorbital, 35 cm2 | 1.25 mA for 11 min. | Each participant received four sessions, each session included one sham and one active tDCS | Nine minutes after real and sham stimulation; | Anodal stimulation of the temporal lobe increases the amplitude of auditory P50, cathodal stimulation of the temporo-parietal region increases the amplitude of N1 |
| Terada, et al., 2015 [31] | 10 | tDCS: anodal/cathodal/sham | F3, 35 cm2 | right mastoid, 35 cm2 | 1.0 mA for 15 min | Three stimulation sessions | The entire experiment process is about 60 min | Cathodal tDCS significantly changed auditory P50 sensory gating index Feeling gating may be regulated by the cathodal tDCS of the left DLPFC, not by the anodal/sham tDCS |
| Kunzelmann, et al., 2018 [35] | 24 | tDCS: anodal/sham | over TP7 and P7, 35 cm2 | over Fp2, AF4, and AF8 25 cm2 | 1.0 mA for 20 min | Two stimulation sessions | The entire experiment process is about 60 min | Different stimulations have no significant effect on AEP, and there is no significant difference between different time points Electrode montage location, stimulation intensity, duration, and different stimulation conditions may have a significant influence on the experimental results |
| Boroda, et al., 2020 [37] | 22 | tDCS: anodal/sham | T7 and T8, 3.14 cm2 PiStim electrode | Fp1 and Fp2 3.14 cm2 PiStim electrode | 1.0 mA for 5 min | Two stimulation sessions | The entire experiment process is about 60 min | Regardless of tDCS, during the target pitch, the N100 component is enhanced compared to the baseline, and there is no difference during the control pitch Compared with sham stimulation, tDCS regulates the plasticity of the target sound |
| Chen, et al., 2014 [41] | 10 | tDCS: anodal/cathodal/sham/no stimulation | F4, 35 cm2 | Left supraorbital unspecified size | 2 mA for 25 min. | Four stimulation sessions | 19 min after stimulation. | The anodal tDCS on the right DLPFC reduced the frequency deviation of auditory MMN, but there was no change for the duration deviation |
| Weigl, et al., 2016 [44] | 18 | tDCS: anodal/cathodal/sham | F3, 35 cm2 | right supraorbital 35 cm2 | 1 mA for 15 min | Three stimulation sessions | Thirty-five minutes before and after stimulation | The anodal tDCS on the left DLPFC can reduce the time course and intensity deviation of auditory MMN, but does not affect the frequency deviation |
| Impey and Knott 2015 [42] | 12 | tDCS: anodal/sham | C5, T7, unspecified size | Contralateral forehead, unspecified size | 2 mA for 20 min | Two stimulation sessions | Unspecified | After the anodal tDCS, the MMN amplitude caused by the small tone is increased The anodal tDCS on the auditory cortex can increase the hearing discrimination ability of healthy subjects |
| Royal, et al., 2018 [46] | 14 | tDCS: frontal, temporal, sham | between electrodes Fp1, AF3 and AF7, 35 cm2 | (1) frontal: between the AF8 and F8, (2) temporal: between the T8 and TP8, (3) Sham: random, 35 cm2 | 2 mA for 20 min | Three stimulation sessions | 16 min before stimulation and 32 min after stimulation | The amplitude of P3 in the frontal and temporal regions after cathodal stimulation was lower than the baseline before stimulation The areas around tDCS inferior frontal gyrus and auditory cortex can induce temporary changes in brain activity induced when dealing with pitch deviations |
| Van Doren, et al., 2014 [53] | 14 | tRNS/sham | Simultaneous T7 and T8; 35 cm2 | unspecified | 2 mA for 20 min | Two stimulation sessions | Twelve minutes before and after stimulation. | The ASSR caused by the frequency modulation tone of 40 Hz has increased significantly TRNS can induce increased excitability in the auditory cortex |
| Miyagishi, et al., 2018 [54] | 24 | tDCS: left anodal, right cathodal/sham | Simultaneous F3 and F4; 35 cm2 | unspecified | 2 mA for 13 min | Two stimulation sessions | MEG test method | tDCS has no significant effect on the ASSR in the γ band Enhanced β-band ERSP of the left motor cortex after tDCS |
| Pellegrino, et al., 2019 [55] | 15 | tDCS: left anodal, right cathodal/sham | Simultaneous C3 and C4, 35 cm2 | unspecified | 2 mA for 20 min | Two stimulation sessions | MEG test method | Since the most affected area is far away from the stimulation point, tDCS has a strong remote effect tDCS will significantly reduce the γ synchronization of the auditory cortex |
| Hyvarinen, et al., 2018 [57] | 18 | Sine 12 Hz-tACS, 6.5 Hz-tACS no stimulation | Simultaneous T3 and T4, 35 cm2 | unspecified | (1) no stimulation:10 min (2)12 Hz-tACS: 1.5 mA for 10 min; (3) 6.5 Hz-tACS: 1.5 mA 2 min | 6.5 Hz-tACS and 12 Hz-tACS twice each. | MEG test method | Compared with no stimulation, the power of ASSR is significantly reduced under the tACS condition of 12 Hz, while the ASSR of 6.5 Hz-tACS is not reduced |
| Reference | Sample Size | Stimulation Types | Stimulation Position and Electrode Size | Reference Electrode Position and Size | Stimulation Parameters | The Number of Stimulation Sessions | EEG Recording Time | Auditory Paradigm | Results |
|---|---|---|---|---|---|---|---|---|---|
| Ladeira, et al., 2011 [61] | 11 | tDCS: anodal/cathodal/sham | Simultaneous T3 and T4, 35 cm2 | Contralateral deltoid muscle 35 cm2 | 2.0 mA for 10 min | Three stimulation sessions | unspecified | Random gap detection test | Anodal tDCS enhanced temporal resolution and cathodal diminished temporal resolution |
| Heimrath, et al., 2014 [62] | 15 | tDCS: anodal/sham | T7, T8, 25 cm2 | Contralateral C4, C3, 50 cm2 | 1.5 mA for 13 min | Two stimulation sessions | unspecified | Between channel gap detection task | Anodal tDCS over the left auditory cortex diminished temporal resolution |
| Baltus, et al., 2018 [65] | 26 | IGF ± 4 Hz tACS | Left hemispheric: FC5 and TP7/P7 Right hemispheric: FC6 and TP8/P8 Each hemispheric two round electrodes (2.5 cm diameter) | unspecified | 1.0 mA for 7 min | Each participant had one stimulation session within two days | Day 1: 40 min in the IGF estimation. Day 2: 1.5 min in the aftereffect estimation. | Between channel gap detection task | Participants in Group A (tACS frequency above IGF) performed significantly better during tACS compared to participants in Group B (tACS frequency below IGF) Both groups had significant relative changes in the amplitude of ASSR |
| Baltus, et al., 2020 [66] | 16 | IGF + 3 Hz tACS or IGF − 4 Hz tACS | Left hemispheric: FC5 and TP7/P7 Right hemispheric: FC4 and TP8/P8 Each hemispheric two round electrodes (2.5 cm diameter) | unspecified | 1.0 mA for 8.5 ± 1.7 min | Each participant had two stimulation sessions within two days | Day 1: 20 min in the IGF estimation. | Between channel gap detection task | The IGF was significantly related to the gap detection performance under the condition that the baseline and tACS frequency were higher than IGF 3 Hz Under the condition that the tACS frequency was higher than IGF 3 Hz, tACS could modulated gap detection performance |
| Rufener, et al., 2017a [67] | 20 | HF-tRNS (100–640 Hz)/sham | Over T7 and T8 35 cm2 | unspecified | 1.5 mA for 40 min | Two stimulation sessions. Stimulation twice in each session. | Record EEG during gap task and pitch task. Unspecified time. | Between channel gap detection task and pitch discrimination task. | Auditory tRNS only increased the detection rate in the temporal domain, no such effect on the discrimination of spectral features The facilitation effect of tRNS was limited to the processing of stimuli near the threshold |
| Rufener, et al., 2017b [68] | 18 | tRNS/sham unspecified frequency | LAC, RAC, BAC unspecified size | unspecified | 1.5 mA for 30 min | Four stimulation sessions. | tRNS simultaneous EEG About 30 min | Between channel gap detection task and pitch discrimination task. | The application of tRNS on LAC and BAC was related to task performance LAC had functional relevance when dealing with temporal features, and this effect was modulated by the the inter-tone interval between the tone triplets |
| Lewald 2016 [70] | 74 | tDCS: anodal and cathodal/sham | (1) superior temporal gyrus (STG, n = 24) (2) inferior parietal lobule (IPL, n = 28) (3) somatosensory-motor cortex (SMC, n = 22). Two round electrodes (diameter 21 mm, 3.5 cm2) | unspecified | 0.4 mA for 12 min | Each participant had one stimulation session at a different stimulation site | unspecified | Simulated “cocktail-party” situation | The tDCS over superior temporal gyrus could enhance the accuracy of target localization in left hemispace TDCS over IPL and off-target active stimulation over SMC found no significant effects |
| Lewald 2019 [71] | 24 | tDCS: anodal/sham | Midpoint between C5 and T7 locations and C6 and T8 locations 35 cm2 | over the contralateral shoulders 7 × 14 cm | 1.0 mA for 32 min | Two stimulation sessions | unspecified | Simulated “cocktail-party” situation | TDCS over the region of posterior STG, significantly improved the performance in localizing a target speaker Compared with sham tDCS, the average increase in correct responses by 3.7% after active tDCS |
| Hanenberg, et al., 2019 [72] | 39 | tDCS: anodal/cathodal/sham | Midpoint between C6 and T8 35 cm2 | over the contralateral shoulder(left) 7 × 14 cm | 1.0 mA for 16 min | Three stimulation sessions. | Whole session about 91 min; | Simulated “cocktail-party” situation | The amplitude of the response of the N2 component on the contralateral (left) increased significantly within 15 min after tDCS At the same point in time, the posterior parietal cortex (PPC) electrical activity reduced on the same side (right) |
| Malte, et al., 2018 [78] | 20 | 10 Hz tACS and sham or 47 Hz tACS and sham | FC5 and TP7 Round electrodes of 3 cm diameter | unspecified | 1.0 mA for 25 min | Each participant received two sessions, each session included one sham and one real tACS | unspecified | Dichotic listening task | Unihemispheric α-tACS relatively reduced the recall of targets contralateral to stimulation, increased recall of ipsilateral targets Contrary to α-tACS, γ-tACS relatively increased the recall of targets contralateral to stimulation, decreased recall of ipsilateral targets |
| Deng, et al., 2019 [79] | 38 | 10 Hz HD-tACS/6 Hz HD-tACS/sham | P2 ring electrode unspecified size | CP2, P4, Pz, PO4 ring electrode unspecified size | 1.5 mA for 20 min | Each participant received one sham and one HD-tACS | unspecified | Selective auditory attention task | RIPS’ α-HD-tACS disrupts auditory spatial attention for leftward No effect on performance in the myriad control conditions (i.e., gender, continuous/switching experiment, theta stimulation) |
| Rufener, et al., 2018 [82] | 20 | HF-tRNS (100–640 Hz)/sham | F3 25 cm2 | right shoulder 35 cm2 | 1.5 mA for 30 min executed twice per session | Two stimulation sessions. | tRNS simultaneous EEG About 60 min | Oddball paradigm (target tone recognition) | TRNS regulates the excitability of the left DLPFC TRNS reduced the subject’s response time to identify the target sound, and fastened up the latency of the P3 component |
| Reference | Sample Size | Stimulation Types | Stimulation Position and Electrode Size | Reference Electrode Position and Size | Stimulation Parameters | The Number of Stimulation Sessions | EEG Recording Time | Auditory Paradigm | CV Syllables | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Heimrath et al., 2016 [84] | 13 | tDCS: anodal/cathodal/sham | Simultaneous T7 and T8, 25 cm2 | Cz 50 cm2 | 1.5 mA for 22 min. | Three stimulation sessions | Record EEG during CV-task II. Unspecified time. | Phoneme categorization task (CV-task I, II) | I: /da/ or /ta/ II: /ba/, /da/, /ga/ or /pa/, /ta/, /ka/ | Cathodal tDCS improved the speech classification ability of CV-syllables in a VOT continuum After the anodal tDCS, the amplitude of the P50 component of all syllables increased |
| Rufener et al., 2016a [87] | Group 1: n = 21 Group 2: n = 17 | Group 1: 6 Hz-tACS/40 Hz-tACS Group 2: no stimulation | Simultaneous T7 and T8, 35 cm2 | Unspecified | 1.0 mA for 18 min | Two stimulation sessions | Unspecified | Phoneme categorization task (CV-task). | /da/ or /ta/ | Application of 40 Hz-tACS selectively reduced the repetition-induced improvement of phoneme categorization abilities |
| Rufener et al., 2016b [89] | Young subjects: n = 25 Older subjects: n = 20 | 6 Hz-tACS/40 Hz-tACS | Simultaneous T7 and T8, 35 cm2 | Unspecified | 1.0 mA for 8 min | Two stimulation sessions | Unspecified | Phoneme categorization task (CV-task). | /da/ or /ta/ | 40-Hz tACS on the bilateral auditory cortex, it was diminished task accuracy in young adults, older adults improved the accuracy of phonetic categorization |
| Jan et al., 2019 [94] | 26 | 40 Hz-HD-tACS/sham | Bilateral AC eight Ag/AgCl electrodes 12 mm diameter | Unspecified | 1.0 mA for 20 min | Two stimulation sessions | 5 min before and after stimulation; | Phoneme categorization task (CV-task) | Distinguish between /ba/, /da/, /ga/, /pa/, /ta/, /ka/ | The 180° tACS during DL at group level did not affect the right ear advantage The phase asymme-tries inherent in the sham-tACS process determined the directionality of behavioral modulations |
| Prete et al., 2018 [97] | Group 1: n = 50 Group 2: n = 24 | Group 1: bilateral HF-tRNS and sham Group 2: unilateral HF-tRNS and sham | Group 1: Simultaneous T3 and T4. 5 × 9.5 cm2 and 5 × 5 cm2 Group 2: T3, T4. 5 × 5 cm2 | Group 1: unspecified Group 2: contralateral shoulder 5 × 9.5 cm2 | 1.5 mA for 20 min | Two stimulation sessions | Unspecified | Dichotic pairs of CV syllables | Pairs /ba/, /da/, /ga/, /pa/, /ta/, /ka/ | The right ear advantage during sham-stimulation and HF-tRNS The HF-tRNS of the bilateral AC was significantly enhanced the right ear advantage effect compared with the sham stimulation |
| Reference | Sample Size | Stimulation Types | Stimulation Electrode Position and Size | Reference Electrode Position and Size | Stimulation Parameters | The Number of Stimulation Sessions | Auditory Paradigm | TACS Time Lags | TACS Phase Relationships | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Wilsch, et al., 2018 [102] | 19 | Envelope—tACS/sham | Simultaneous Cz (35 cm2) T3 and T4 (4.18 × 4.18 cm) | Unspecified | between 0.4 mA–1.5 mA unspecified time | Two stimulation sessions | Oldenburg sentence test | 0–250 ms, in 50-ms steps | Unspecified | The sinusoidal fit with an average frequency of 5.12 Hz has a good effect on envelope-tACS modulation of speech intelligibility |
| Zoefel, et al., 2020 [103] | Group 1: n = 27 Group 2: n = 19 | Group 1: unilateral tACS and sham Group 2: bilateral tACS and sham | Group 1: T7 (3 × 3 cm) C3 (5 × 7cm). Group 2: T7 and T8 doughnut-shaped electrodes inner ring (d 1 = 2 cm, t 2 = 0.1 cm) external ring (outer d 1 = 10 cm, inner d 1 = 7.5 cm, t 2 = 0.2 cm) | Unspecified | Group 1: 1.7 mA Group 2: 1.2 mA unspecified time | Two stimulation sessions | Speech comprehension test (rhythmic noise-vocoded speech) | 0–280 ms, in 40-ms steps | 0–7π/4, in steps of π/4 | Modulation of speech perception induced by tACS in the case of bilateral stimulation using ring electrodes |
| Kadir, et al., 2020 [105] | 17 | Envelope—tACS/sham | Simultaneous T7 and T8 (35 cm2) | Either side of the Cz (35 cm2) | between 0.2–1.5 mA unspecified time | Two stimulation sessions | Speech comprehension test (SRT assessment) | Fixed lag times of 100 ms and 250 ms | 0–5π/3, in steps of π/3 | Between a short latency of 100 ms and a long latency of 250 ms, the modulation of speech understanding by the phase of the stimulus was different |
| Keshavarzi, et al., 2020 [106] | 18 | Envelope—tACS/sham | Simultaneous T7 and T8 (35 cm2) | Either side of the Cz (35 cm2) | between 0.7 mA-1.3 mA unspecified time | Two stimulation sessions | Speech comprehension test (SRT assessment) | Unspecified | 0–5π/3, in steps of π/3 | The tACS effect speech understanding in the θ band, had no effect on speech understanding in the δ band Compared with the sham stimulation, the current stimulation in the θ band can improve the understanding of speech in noise |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Shi, L.; Dong, G.; Zhang, Z.; Chen, R. Effects of Transcranial Electrical Stimulation on Human Auditory Processing and Behavior—A Review. Brain Sci. 2020, 10, 531. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10080531
Wang Y, Shi L, Dong G, Zhang Z, Chen R. Effects of Transcranial Electrical Stimulation on Human Auditory Processing and Behavior—A Review. Brain Sciences. 2020; 10(8):531. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10080531
Chicago/Turabian StyleWang, Yao, Limeng Shi, Gaoyuan Dong, Zuoying Zhang, and Ruijuan Chen. 2020. "Effects of Transcranial Electrical Stimulation on Human Auditory Processing and Behavior—A Review" Brain Sciences 10, no. 8: 531. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci10080531