1. Introduction
Ischemic stroke is one of the most common causes of severe disability worldwide [
1]. To date, few treatments options are available, and those that are available such as clot busters are only effective if given within a narrow therapeutic time window (4.5–6 h) after stroke onset [
2]. As a consequence, only 5–10% of stroke survivors will receive thrombolysis, leaving the remaining 90–95% dependent on physical therapy to improve their recovery outcomes. Stroke survivors often experience lasting motor impairments; therefore, there is an urgent need to find novel therapies that can be administered days to weeks after stroke to help improve motor function.
Changes in γ-aminobutyric acid (GABA) function following cerebral ischemia and the protective benefits of GABAergic compounds targeting synaptic GABA
A receptors have been reported [
3,
4,
5,
6]. Despite GABA
A receptor agonists showing great promise in animal models of stroke, they have failed to translate into positive clinical outcomes [
7], which is most likely due to a lack of subtype specificity resulting in unwanted side effects, hence limiting their therapeutic potential [
8,
9,
10].
Over the past decade, preclinical research has highlighted several mechanisms related to post-stroke recovery [
11,
12,
13,
14]. Many of these processes involve changes in molecular signaling pathways, which are closely linked to changes in learning and memory [
15] and an imbalance in brain excitability [
5,
16,
17].
By selecting compounds and dosing paradigms that selectively target individual GABA receptor subtypes, researchers have shown that the modulation of either tonic or phasic inhibition can enhance functional motor recovery in rodents after stroke [
5,
18,
19]. GABA
C receptors also known as GABA
A rho (
ρ) receptors are insensitive to bicuculline; they can be found extrasynaptically, and similar to other extrasynaptic GABA
A receptors (e.g., α5- or δ-containing GABA
A receptors), they do not readily desensitize in the presence of GABA [
20,
21]. In addition to modulating tonic inhibitory currents directly by targeting extrasynaptic GABA
A receptors, we have recently also reported that targeting the astrocytic GABA transporter type 3 (GAT3) [
22] using the substrate, L-isoserine, increases the expression of GAT3 and improves motor function [
19].
Given the extrasynaptic location of GABA
C receptors, and as their expression are in regions involved in motor control such as the neostriatum and cerebellum [
23,
24], we envisaged that such receptors could be an interesting target to explore for stroke recovery. As GABA
C receptors are expressed on both neurons and astrocytes [
25,
26,
27], we asked the question whether inhibiting GABA
CRs can also play a role in improving motor function.
(
R)- and (
S)-4-amino-cyclopent-1-enyl butylphosphinic acid ((
R)-4-ACPBPA and (
S)-4-ACPBPA) are compounds that selectively inhibit the
ρ-subunits of GABA
C receptors [
22,
28]. Both compounds are inactive at the α1β2γ2 GABA
A receptors when tested at doses < 600 µM and inactive at the GABA
B receptor at doses < 300 µM [
28]. Furthermore, both compounds have recently been evaluated in vivo and are efficacious at preventing ethanol-induced motor incoordination in mice [
22].
In the present study, we used (
R)-4-ACPBPA and (
S)-4-ACPBPA to target GABA
C receptors and measured changes in functional recovery, and glial markers, glial fibrillary acidic protein (GFAP), and GAT3 expression after inducing photothrombotic stroke. These studies were compared to the α5-subunit negative allosteric modulator (NAM), L655,708, which improves motor recovery preclinically after stroke [
5].
2. Materials and Methods
2.1. Animals
All procedures described in this study were approved by the University of Otago, Animal Ethics Committee in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (AEC #92/11). As GABA
CR modulators have not been tested before in a stroke model, it is widely accepted that all testing be undertaken in young male mice first. A total of 130 young (2–3-month-old) male C57BL/6
J mice were used for these studies, which allows us to compare and validate results to previous published studies. All mice were housed under a 12 h light/12 h dark cycle (lights on 0700 h) with ad libitum access to food and water. All mice were group housed (
n = 3–5/cage) in Tecniplast caging with corncob bedding. Cages were enriched with shredded paper, plastic tunnels, and wood blocks for chewing, with new enrichments replaced weekly. All mice were assigned to either stroke or sham experimental groups at the time of surgery by one member of staff, with both groups being further randomly assigned to a treatment group 3 days later by a second member of staff to ensure all studies were undertaken in a blinded fashion. All samples sizes for the various assessment parameters were calculated based on our own prior studies and the use of sample size calculators [
5,
29].
2.2. Allocation of Animals to Different Experiments
For the qPCR experiment, a total of 30 animals, 5 for each time point (0, 1, 3, 7, 14, and 28 days post-stroke) were used. For behavioral assessments, a total of 100 animals were divided into 10 different groups of 10 animals: (1) Sham + Vehicle, (2) Sham + L655,708, (3) Sham + 5 mM (S)-4-ACPBPA, (4) Sham + 5 mM (R)-4-ACPBPA, (5) Stroke + Vehicle, (6) Stroke + L655,708, (7) Stroke + 2.5 mM (S)-4-ACPBPA, (8) Stroke + 5 mM (S)-4-ACPBPA, (9) Stroke + 2.5 mM (R)-4-ACPBPA, (10) Stroke + 5 mM (R)-4-ACPBPA. Of these animals, six from each group were randomly selected to determine infarct size, GAT3, and GFAP analysis. For the combinatorial studies, a total of 48 animals were divided into 6 different groups of 8 animals: (1) Sham + Vehicle, (2) Sham + 5 mM L655,708 + 5 mM (R)-4-ACPBPA, (3) Stroke + Vehicle, (4) Stroke + L655,708, (5) Stroke + 5 mM (R)-4-ACPBPA, (6) Stroke + 5 mM L655,708 + 5 mM (R)-4-ACPBPA.
2.3. Photothrombosis Model of Focal Ischemia
Focal stroke was induced in the left hemisphere using the photothrombosis method in young adult (2–3 month old, 27–30 g) male C57BL/6
J mice [
5,
29]. In brief, under isoflurane anesthesia (2–2.5% in medical O
2), mice were placed in a stereotaxic apparatus, the skull exposed and a cold light source (KL1500 LCD, Carl Zeiss, Auckland, New Zealand) attached to a 20× objective to give a 2 mm diameter illumination positioned 1.5 mm lateral from Bregma. Animals were administered 0.2 mL of Rose Bengal solution (Sigma-Aldrich, Auckland, New Zealand; 10 g/L in normal saline) intraperitoneally (i.p.) and left for five minutes to allow circulation prior to a 15 min illumination of the brain. Body temperature was maintained at 36.9 ± 0.3 °C with a heating pad (Harvard apparatus, Holliston, MA, USA) throughout surgical procedures. Following the light exposure, the skin was closed using surgical glue. After a brief recovery period, animals were returned to their normal housing conditions. Sham animals received saline instead of Rose Bengal solution.
Animals were randomly allocated to different treatment groups: L655,708 (5 mM pump concentration), (
R)-4-ACPBPA (2.5 mM and 5 mM pump concentrations), (
S)-4-ACPBPA (2.5 mM and 5 mM pump concentrations), combined L655,708 and (
R)-4-ACPBPA (5 mM pump concentration each) or vehicle. Drugs were dissolved in DMSO and then diluted 1:1 in 0.9% saline. Vehicle-treated animals were implanted with pumps filled with 1:1 DMSO and 0.9% saline solution. All compounds have previously been tested in vivo and shown to cross the BBB and have an effect on the brain, including L655,708, which we have previously reported is effective after stroke [
5].
Drug or vehicle-filled ALZET-1002 pumps (DURECT Corporation: Cupertino, CA, USA) were implanted on day 3 post-stroke and replaced every two weeks [
5]. The concentration in one minipump, 5 mM, delivers a 200 µg/kg/day dose in mice. To implant the ALZET-1002 pumps, mice were anesthetized with isoflurane/O
2 mixture, and a small (<1 cm) incision was made between the shoulder blades of the animals. A subcutaneous pocket was made by gently inserting blunt forceps under skin. The pump was placed with port side facing away from the incision and the incision closed using surgical glue. During all surgical procedures, mice received temgesic as pain relief on the day of surgery as well as the following day. In addition, for minipump implantation procedures, topical lidocaine was also given.
To ensure that all experiments were undertaken in a blind manner, animals were allocated into each of the treatment groups, and minipumps were implanted by someone not undertaking any of the assessments (behavior, real-time qPCR, immunohistochemical, and histological stains). For this, animals in each cage were assigned a number, and then each number was randomly assigned to a treatment.
2.4. Tissue Collection
All mice at the end of the study were sacrificed by anesthetic overdose with pentobarbital (100 mg/kg). For tissue being collected for qPCR analysis, brains were rapidly removed from the cranium, frozen on dry ice, and stored at −80 °C prior to being used. All other mice underwent transcardiac perfusion with 4% formaldehyde, and brains were removed for histochemical assessments.
2.5. Real-Time qPCR Measurement of GABA Subunits (n = 5 at Each Time Point)
The relative mRNA expression of GABA subunits (α5,
ρ1 and
ρ2) were assessed by quantitative real-time polymerase chain reaction (qPCR) [
17] in
n = 5 mice at each time point. Bain tissue samples that included both the stroke and surrounding peri-infarct area were collected and snap-frozen at 0 °C (control non-stroked tissue), 1, 3, 7, 14, or 28-days post-stroke. Total RNA was extracted using the Qiagen RNeasy kit and following the manufacturer’s protocols. The purity (RNA with ratio of absorbance at 260 nm and 280 nm ≥ 2) and amount of the RNA was measured spectrophotometrically (NanoDrop 2000, Thermo Scientific, Waltham, MA, USA). Total RNA (750 ng) was used to synthesize the first-strand complementary DNA (cDNA) using Super Script III (Life Technologies, Waltham, MA, USA) following the manufacturer’s protocol. After reverse transcription, the cDNA was amplified by qPCR using SyBr green master mix (Applied Biosystems, Foster City, USA) and each of the following primer (250 nM) sets; α5: forward—GCTGACCCATCCTCCAAACA, reverse—TGGAGACTGTGGGTGCATTC;
ρ1: forward—CTTCTCACGGCTTCTTGGGA, reverse—ACCCATCCCCACCACAAAAG;
ρ2: forward—CCATTAAAAGTCCCTGCACAGC, reverse—ATGTTTCCAGAAGCCCTGTCC; Rpl13a: forward—ATTGTGGCCAAGCAGGTACT, reverse—CTCGGGAGGGGTTGGTATTC. qPCR was performed on a Roche Lightcycler 480 (Roche, Minneapolis, USA) with the following cycling parameters: 40 cycles of 95 °C, 15 s; 60 °C, 30 s; 72 °C, 40 s. After amplification, a denaturing curve was performed to ensure the presence of unique amplification products. All reactions were performed in triplicate. Expression of mRNA was assessed by evaluating threshold cycle (CT) values. The CT values were normalized against the expression level of the house keeping gene succinate dehydrogenase complex flavoprotein complex A (SDHA).
2.6. Electrophysiology in Xenopus laevis Oocytes
All procedures using Xenopus laevis were approved by the animal ethics committee of the University of Sydney (AEC No. 2013/5269). Female Xenopus laevis of approximately 1 year of age were from Xenopus Express Brookville, FL, USA. To obtain isolated oocytes, ovarian lobes were removed from anesthetized adult female frogs incised into small pieces using surgical scissors and defolliculated by collagenase A treatment. Stage V and VI oocytes were injected with cRNA mixture encoding α5, β2, and γ2 at a ratio of 5:1:5, 25 ng/cell, or 50 ng/cell ρ2 cRNA GABAAR subunits and were incubated at 18 °C for 3–5 days in ND96 solution (96 mM NaCl, 2.0 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 0.5 mM theophylline, and 50 µg/mL gentamicin; pH 7.4) before electrophysiological experiments. Stock solutions of GABA (1 mM), (S)-4-ACPBPA (100 mM), and (R)-4-ACPBPA (100 mM) were prepared by dissolving solid compounds in ultrapure water.
Whole-cell currents were recorded using the two-electrode voltage clamp technique. To determine the GABA inhibitory activity of (S)-4-ACPBPA and (R)-4-ACPBPA on ρ2 receptors, inhibitory concentration response curves of (S)-4-ACPBPA and (R)-4-ACPBPA were co-applied with GABA 1 µM (≈EC50) with oocytes clamped at −60 mV. Data were acquired with a LabChart v 3.5.2 (ADInstruments Pty Ltd, New South Wales, Australia) analogue to digital converter, and currents low-pass-filtered at 1 kHz and sampled at 3 kHz were measured offline with LabChart v3.5.2 software. The bath solution contained ND96 solution, and electrodes were filled with 3M KCl (0.5–2 MΩ). Solutions were bath applied using a gravity-fed perfusion system.
To determine the GABA inhibitory activity of (S)-4-ACPBPA and (R)-4-ACPBPA on α5β2γ2 receptors, two concentrations of (S)-4-ACPBPA and (R)-4-ACPBPA, 10 µM and 100 µM, were co-applied with GABA 10 µM (≈EC30). To ensure the reproducibility of the evoked current amplitudes, a set of control applications was performed before the inhibitory experiments: three GABAcontrol (10 µM) applications, one GABAmax (316 µM approximately EC100) application, and another three GABAcontrol (10 µM) applications. This is followed by the co-application of GABA control (10 µM) with (S)-4-ACPBPA (10 µM) or (R)-4-ACPBPA (10 µM), then GABA control (10 µM) to ensure the current return to previous control level, and then the co-application of GABA control (10 µM) with (S)-4-ACPBPA (100 µM) or (R)-4-ACPBPA (100 µM). Data were assembled from a minimum of 6 independent experiments (n = 6) with errors expressed as SEM.
2.7. Behavioral Assessment (n = 8–10/Group)
Animals were tested one week prior to surgery on both the grid-walking and cylinder tasks to establish baseline performance levels. Then, all animals were tested on weeks 1, 2, 3, 4, and 6 weeks post-stroke at approximately the same time each day, at the end of their dark cycle. All behaviors were scored by observers who were blind to the treatment group of the animals in the study as previously described [
5,
16]. Ten animals per group were assessed on all behavioral tasks, except for the combinatorial studies, where we used an
n = 8/group.
2.8. Grid-Walking Test
The grid-walking apparatus was manufactured using 12 mm square wire mesh with a grid area 32 cm/20 cm/50 cm (length/width/height). A mirror is placed beneath the apparatus to allow video footage in order to assess the animals’ stepping errors (i.e., “foot-faults”). Each mouse is placed individually atop of the elevated wire grid and allowed to freely walk for a period of 5 min (measured in real time by stopwatch and confirmed afterwards by reviewing videotape footage). During this 5-min period, the total number of foot-faults for each limb along with the total number of non-foot-fault steps are counted, and a ratio between foot-faults and total steps taken was calculated. The percent of foot-faults was calculated as follows: (#foot-faults/(#foot-faults + #non-foot-fault steps) * 100). A ratio between foot-faults and total steps taken was used to take into account differences in the degree of locomotion between animals and trials.
2.9. Cylinder Task
The spontaneous forelimb task encourages the use of forelimbs for vertical wall exploration/press in a cylinder. When placed in a cylinder, the animal rears to a standing position, whilst supporting its weight with either one or both of its forelimbs on the side of the cylinder wall. A cylinder 15 cm in height with a diameter of 10 cm is used. Videotape footage of animals in the cylinder is evaluated quantitatively in order to determine forelimb preference during vertical exploratory movements. While the video footage is played in slow motion (1/5th real time speed), the time (s) during each rear that each animal spent on either the right forelimb, the left forelimb, or on both forelimbs are calculated. Only rears in which both forelimbs could be clearly seen are timed. From these three measures, the total amount of time spent on either limb independently as well as the time the animal spent rearing using both limbs was derived. The percentage of time spent on each limb was calculated, and these data were used to derive a spontaneous forelimb asymmetry index (% ipsilateral use/% contralateral use). The “contact time” method of examining the behavior was chosen over the “contact placement” method, as it takes into account the slips that often occur during a bilateral wall press post-stroke.
2.10. Immunohistochemical and Histological Assessments (n = 6)
Following the final behavioral assessment at 6 weeks post stroke, all animals were anesthetized and transcardially perfused with 4% paraformaldehyde. Brains were extracted, and 30 μm thick coronal sections were collected using a sliding microtome. Then, tissue was processed histologically using cresyl violet staining in order to quantify infarct volume as previously described [
5,
16,
19,
30]. Images of cresyl violet staining were taken using an inverted montaging microscope (Model Ti2E Wideview, Nikon, Japan) set with a 2.5× objective lens. Then, images were exported as TIFF files and opened on Fiji ImageJ to quantify infarct volume. Stroke volume was calculated as per the equation:
Immunofluorescent labeling of GAT3 and GFAP was performed 42 days post stroke to assess the effects of GABA treatments on glial scar formation and the astrocytic GABA transporter, respectively. Briefly, sections were washed thoroughly in Tris-buffered saline (TBS), blocked in 5% donkey serum, and incubated for 48 h at 4 °C in either the polyclonal chicken anti-GFAP (1:2000; Millipore, Burlington, MA, USA) or in the rabbit anti-mouse GAT3 (1:100, Millipore, Burlington, MA, USA) primary antibodies, which were diluted in TBS containing 0.3% Triton X-100 and 0.25% bovine serum albumin (hereafter referred to as incubation solution) containing 2% normal donkey serum. Then, sections were washed three times in TBS (10 min per wash) before being incubated in either the donkey anti-chicken 549 secondary antibody (1:400; Jackson Immunoresearch, West Grove, PA, USA) or the donkey anti-mouse 488 DyLight (1:400; Sapphire Bioscience, New South Wales, Australia) secondary antibody in incubation solution for 90 min at room temperature. After subsequent washing in TBS, sections were mounted onto gelatin-coated glass slides, air-dried, and passed sequentially through alcohols (50%, 70%, 95%, and 100%) before being passed through xylene and then cover slipped using DPX mounting solution.
Images of the glial scar (400 µm from the stroke border) encompassing what is known as the peri-infarct region were taken on an Olympus BX61 (Olympus, Auckland, New Zealand) microscope using a QImaging camera (Micropublisher 5.0 RTV, 2560 × 1920 pixels, 3.4 µm pixel size; Olympus, Auckland, New Zealand) and 20× UPlanSApo objective (0.75NA: Olympus, Auckland, New Zealand). Fluorescent intensity measures were taken using ImageJ (National Institutes of Health, Bethesda, MA, USA) analysis. Fluorescent intensity measurements of the scar were normalized to background reading on the contralateral hemisphere, and an average fluorescence was obtained using 3 sections per animal (n = 6). Images and fluorescent intensity measurements were conducted by observers blinded to treatment group allocation.
2.11. Statistical Analysis
All statistical analyses were performed using GraphPad Prism (version 7.0c, San Diego, CA, USA). One- or two-way analysis of variance (ANOVA) and Tukey’s multiple pair-wise comparisons for post hoc comparisons were used. Electrophysiological data are represented as mean ± SEM, all other data as mean ± SD, and p < 0.05 was considered statistically significant.
2.12. Power Analysis
For behavioral experiments, 6 animals per group are required to achieve >80% power (86% calculated), considering the following parameters: α = 0.05; with an effect size = 1.5. For qPCR and histological/immunohistochemical experiments, 5 animals per group are required to achieve >80% power (91% calculated), considering the following parameters: α = 0.05; effect size 1; 3 concentrations; 2 groups, and correlation between measures =0.5. Parameters were determined from our prior work, in which we have demonstrated significant behavioral effects [
5,
17,
19,
31], and on the assumption that variance was about 25%. It should be noted that more conservative effect sizes were used for these experiments, as it is harder to assess recovery over time between groups than looking at the effects of drug treatments on stroke size.
4. Discussion
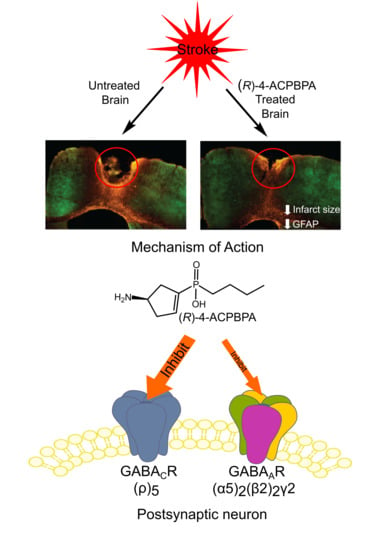
The current study investigated whether targeting GABA
CRs can promote motor recovery after stroke, as these receptors have previously been reported to mediate tonic inhibition and are located in brain regions important for motor control [
21,
27]. We show that GABA
C-
ρ2 relative mRNA expression increased in the peri-infarct region 3 and 7 days after stroke, with GABA
C-ρ1 also showing an increase 7-days after stroke, although it is not clear whether these increases result in functional changes. Therefore, in order to test whether GABA
C ρ-containing receptors play a role in motor recovery after stroke, we evaluated the
ρ-antagonists (
S)-4-ACPBPA and (
R)-4-APCBPA in a photothrombotic mouse model of stroke. L655,708, the GABA
A α5 NAM, was used as a positive control, as inhibiting tonic currents mediated by α5-containing receptors improves motor function from 3 days post-stroke [
5,
32].
All three compounds, L655,708, (
S)-4-ACPBPA, and (
R)-4-ACPBPA improved motor function from 3 days post stroke. On the grid-walking task, (
R)-4-ACBPBA was more effective than the
S-enantiomer, observing a dose-dependent improvement in performance (at 2.5 and 5 mM); however, these mice never reached the same level of performance as mice treated with L655,708. Measuring the use of the impaired limb using the cylinder task, it is clear that L655,708 was more effective than (
R)-4-ACPBPA. The
S-enantiomer was only effective in improving performance on the grid-walking test, and this was only observed at the highest dose tested. No significant effect was observed at the lower dose. Furthermore, (
R)-4-ACPBPA but not (
S)-4-ACPBPA or L655,708 decreased the expression of GFAP positive reactive astrocytes and increased GAT3 expression. In contrast, infarct volume was unchanged in all treatment groups, indicating that when treatment is started from 3 days post-stroke, the infarct is already fully formed and any behavioral improvements are the result of brain plasticity. A possible explanation for the differences in GABA
A and GABA
C-mediated functional recovery is that the expression of the GABA
A α5-subunit is higher than that of the GABA
C ρ1 and
ρ2-subunits. At the time of undertaking these experiments, we did not realize this was going to be an issue, as the expression of the α5-subunit is low compared to the expression of most other GABA subunits and that the receptor occupancy achieved following 5 mM L655,708 dosing via ALZET osmotic pumps is about 9–14% receptor occupancy ([
5], and unpublished data). Given the relatively low receptor occupancy required to achieve a functional improvement when targeting the α5-subunit with L655,708, it is plausible that some of the effects observed with either (
R)-4-ACPBPA or (
S)-4-ACPBPA could be occurring via the α5-subunit. However, we believe that this is not the case, as (
R)-4-ACPBPA was the only compound to show any glial effects.
Neither compound acts on the synaptic α1β2γ2 GABA
A receptor nor do they activate or inhibit GABA
B receptors at the doses tested [
28]. However, (
S)-4-ACPBPA and (
R)-4-APCBPA differ in their affinity to act as an antagonist at the
ρ2 subtype; (
R)-4-APCBPA was more effective at inhibiting
ρ2, and this provides some insight toward understanding the role that these subunits play in motor recovery. Both L655,708 and (
R)-4-APCBPA afford an early improvement in motor functional recovery, which is a mechanistic process that we have only ever observed via a change in tonic inhibitory currents [
5]. Given that the modulation of GABA
A and GABA
C receptors improves motor function via different mechanisms, we chose to next perform a combinatorial study, which we show resulted in a marked improvement in motor recovery that was better than L655,708 or (
R)-4-APCBPA alone. The only difference being that the recovery trajectory in the combined group is slower across time than that of the L655,708 treatment group; however, it is possible that we are reaching the maximum possible recovery achievable in these animals. The observed synergistic effects of combined dosing with L655,708 and (
R)-4-ACPBPA support our opinion that (
R)-4-ACPBPA is acting on the GABA
C ρ2-subunit containing extrasynaptic receptors, as dosing with α5-subunit selective compounds that achieve either 8% or 60% receptor occupancy results in the same level of motor functional recovery (unpublished data).
The persistent elevation of tonic currents seen in the peri-infarct area after stroke is due to a lack of GAT3 function [
5,
19]. GAT3 is a GABA transporter located on astrocytes that removes GABA from the extrasynaptic space [
33]. Without GAT3, GABA remains in the extrasynaptic space and continues to activate extrasynaptic receptors, resulting in the persistence of tonic currents. It is thought that the increase in tonic inhibition is an intrinsic mechanism that helps limit the spread of damage in the brain and silences communication so the brain can repair itself [
5,
17]. However, this increase in tonic inhibition is limiting the recovery of lost functions, as it remains elevated and chronically silences connections, and it becomes detrimental for stroke recovery, since it makes it harder for neurons to form novel connections and contribute to the plasticity necessary to improve motor functioning [
15]. While the current study did not measure tonic currents, the increase in GAT3 expression in the peri-infarct area after treatment with 5 mM (
R)-4-ACPBPA indicates that the functional improvements seen on grid-walking and cylinder tests may be the result of decreased tonic inhibition and enhanced neuroplasticity. This is similar to what we have previously observed following treatment with either L-isoserine [
19] that resulted in an increase in GAT3 expression and improved functional recovery, and also following L655,708 treatment that dampened that stroke-induced elevation in tonic inhibition via GABA
A α5 receptors [
5,
32]. In addition to the GAT3-mediated increases in tonic inhibitory currents, previous research has also reported that reactive astrocytes produce abnormally large amounts of GABA due to aberrant monoamine oxidase B (MAOB) activity that results in excess GABA being released via Bestrophin-1 (Best-1) channels [
34]. As GABA
C receptors are expressed on astrocytes [
25,
26,
27], it is possible that inhibiting the production and therefore release of GABA from astrocytes via dampening GABA
C receptor activity could also contribute to a decrease in astrocyte reactivity, decrease in tonic inhibition, and increase in GAT3 expression and restoration of peri-infarct synaptic plasticity.
After stroke, astrocytes undergo many morphological changes to form a glial scar; these reactive astrocytes increasingly express GFAP, and this is used as a measure to determine the number and or size of the astrocytes. The glial scar has many roles after a stroke has occurred, both as a regulator of the inflammatory response and in relation to axonal sprouting, which is critical for cortical remapping [
13,
35,
36,
37]. The exact role that reactive astrocytes play after stroke still remains to be fully elucidated, although reactive astrocytes are known to be both beneficial and detrimental during different phases of stroke recovery [
13,
38]. For instance, the glial scar prevents damage from spreading throughout the brain; however, these reactive astrocytes also express a variety of neurite-growth inhibitors such as ephrin-A5, which limits functional recovery [
39].
Here, we see a decrease in GFAP fluorescence after 5 mM (
R)-4-ACPBPA, indicating a decrease in reactive astrocytes in this area. We hypothesize that the decrease of reactive astrocytes is beneficial, given that it correlates with functional recovery. This seems to be in contrast with the increase in GAT3 expression; however, after stroke, GAT3 expression shifts from being almost exclusively on astrocytes to neurons [
40]. The mechanism through which (
R)-4-ACPBPA is decreasing the number or size of the reactive astrocytes seems to be via GABA
C ρ2 receptor specific inhibition given that L655,708 was without effect.
Recommendations for preclinical stroke recovery studies demand different approaches than those for stroke neuroprotection, as stroke recovery studies are concerned with long-term recovery. For this, it is not ideal for stroke models to produce substantial animal death, such as what is observed using the filament occlusion middle cerebral artery models, and models should result in chronic behavioral deficits. These two simple criteria have guided consensus groups to make recommendations for using models that create focal lesions of similar size to those observed in humans [
41]. The photothrombotic stroke model is reproducible, relatively non-invasive, and technically simple, and it is a model recommended by the stroke research recovery roundtable as being ideal for stroke recovery studies [
41]. Disadvantages include a smaller rim of partially damaged peri-infarct cortex, simultaneous multivessel occlusion, and in some animals, the presence of edema, which is not commonly observed in human stroke. Still, the lesion can be positioned in any cortical location targeting particular deficits, and it is useful for assessing mechanistic and pharmacological studies of recovery.