A Retrospective Analysis on Clinical Practice-Based Approaches Using Zolpidem and Lorazepam in Disorders of Consciousness
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Overall Results of the Zolpidem and Lorazepam Trials
3.2. Following a Positive Trial
3.3. Following an Equivocal or a Negative Trial
4. Discussion
4.1. Insights on Clinical Practice and Future Studies
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
References
- Kothari, S. Chronic disorders of consciousness. In Neuropalliative Care: A Guide to Improving the Lives of Patients and Families Affected by Neurologic Disease, 1st ed.; Creutzfeldt, C.J., Kluger, B.M., Holloway, R.G., Eds.; Springer: Cham, Switzerland, 2019; pp. 37–58. [Google Scholar]
- Edlow, B.L.; Claassen, J.; Schiff, N.D.; Greer, D.M. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat. Rev. Neurol. 2021, 17, 135–156. [Google Scholar] [CrossRef]
- Giacino, J.T.; Whyte, J.; Bagiella, E.; Kalmar, K.; Childs, N.; Khademi, A.; Eifert, B.; Long, D.; Katz, D.I.; Cho, S.; et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 2012, 366, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Clauss, R.P.; Güldenpfennig, W.M.; Nel, H.W.; Sathekge, M.M.; Venkannagari, R.R. Extraordinary arousal from semi-comatose state on zolpidem. A case report. S. Afr. Med. J. 2000, 90, 68–72. [Google Scholar]
- Brefel-Courbon, C.; Payoux, P.; Ory, F.; Sommet, A.; Slaoui, T.; Raboyeau, G.; Ma, B.L.; Puel, M.; Montastruc, J.-L.; Demonet, J.-F.; et al. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann. Neurol. 2007, 62, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T.; Conte, M.M.; Goldfine, A.M.; Noirhomme, Q.; Gosseries, O.; Thonnard, M.; Beattie, B.; Hersh, J.; Katz, D.I.; Victor, J.D.; et al. Common resting brain dynamics indicate a possible mechanism underlying zolpidem response in severely brain-injured subjects. eLife 2013, 2, e01157. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.; Duong, T.T. Increased arousal in a patient with anoxic brain injury after administration of zolpidem. Am. J. Phys. Med. Rehabil. 2008, 87, 229–231. [Google Scholar] [CrossRef]
- Shames, J.L.; Ring, H. Transient reversal of anoxic brain injury-related minimally conscious state after zolpidem administration: A case report. Arch. Phys. Med. Rehabil. 2008, 89, 386–388. [Google Scholar] [CrossRef]
- Arnts, H.; van Erp, W.S.; Boon, L.I.; Bosman, C.A.; Admiraal, M.M.; Schrantee, A.; Pennartz, C.M.; Schuurman, R.; Stam, C.J.; van Rootselaar, A.-F.; et al. Awakening after a sleeping pill: Restoring functional brain networks after severe brain injury. Cortex 2020, 132, 135–146. [Google Scholar] [CrossRef]
- Whyte, J.; Myers, R. Incidence of clinically significant responses to zolpidem among patients with disorders of consciousness: A preliminary placebo controlled trial. Am. J. Phys. Med. Rehabil. 2009, 88, 410–418. [Google Scholar] [CrossRef]
- Whyte, J.; Rajan, R.; Rosenbaum, A.; Katz, D.; Kalmar, K.; Seel, R.; Greenwald, B.; Zafonte, R.; Demarest, D.; Brunner, R.; et al. Zolpidem and restoration of consciousness. Am. J. Phys. Med. Rehabil. 2014, 93, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Thonnard, M.; Gosseries, O.; Demertzi, A.; Lugo, Z.; Vanhaudenhuyse, A.; Bruno, M.-A.; Chatelle, C.; Thibaut, A.; Charland-Verville, V.; Habbal, D.; et al. Effect of zolpidem in chronic disorders of consciousness: A prospective open-label study. Funct. Neurol. 2013, 28, 259–264. [Google Scholar]
- Luz, J.; Jang, E.J. Poster 354 lorazepam trial for a patient with a disorder of consciousness: A case report. PM&R 2014, 6, S309. [Google Scholar]
- Mancuso, C.E.; Tanzi, M.G.; Gabay, M. Paradoxical reactions to benzodiazepines: Literature review and treatment options. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 1177–1185. [Google Scholar] [CrossRef]
- Interlandi, J. A Drug that Wakes the Near Dead. The New York Times Magazine. 1 December 2011. Available online: https://www.nytimes.com/2011/12/04/magazine/can-ambien-wake-minimally-conscious.html (accessed on 27 February 2021).
- Du, B.; Shan, A.; Zhang, Y.; Zhong, X.; Chen, D.; Cai, K. Zolpidem arouses patients in vegetative state after brain injury: Quantitative evaluation and indications. Am. J. Med. Sci. 2014, 347, 178–182. [Google Scholar] [CrossRef]
- Bomalaski, M.N.; Claflin, E.S.; Townsend, W.; Peterson, M. Zolpidem for the treatment of neurologic disorders. JAMA Neurol. 2017, 74, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Karri, J.; O’Brien, K.; DiTommaso, C.; Kothari, S.; Li, S. Spasticity management in persons with disorders of consciousness. PM&R 2020. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, K.; Karri, J.; O’Brien, K.; DiTommaso, C.; Li, S. Many faces of the hidden souls: Medical and neurological complications and comorbidities in disorders of consciousness. Brain Sci. 2021, 11, 608. [Google Scholar] [CrossRef]
- Nutt, D. GABAA receptors: Subtypes, regional distribution, and function. J. Clin. Sleep Med. 2006, 2, S7–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA imbalance following traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sayadnasiri, M.; Rezvani, F. Treatment of catatonia in frontotemporal dementia: A lesson from zolpidem test. Clin. Neuropharmacol. 2019, 42, 186–187. [Google Scholar] [CrossRef]
- Cohen, L.; Chaaban, B.; Habert, M.-O. Transient improvement of aphasia with zolpidem. N. Engl. J. Med. 2004, 350, 949–950. [Google Scholar] [CrossRef] [Green Version]
- Esienaert, P.; Dhossche, D.M.; Evancampfort, D.; Hert, M.E.; Gazdag, G. A clinical review of the treatment of catatonia. Front. Psychiatry 2014, 5, 181. [Google Scholar] [CrossRef]
- Sutton, J.A.; Clauss, R.P. A review of the evidence of zolpidem efficacy in neurological disability after brain damage due to stroke, trauma and hypoxia: A justification of further clinical trials. Brain Inj. 2017, 31, 1019–1027. [Google Scholar] [CrossRef]
- Machado, C.; Estévez, M.; Rodríguez, R.; Pérez-Nellar, J.; Chinchilla, M.; DeFina, P.; Leisman, G.; Carrick, F.R.; Melillo, R.; Schiavi, A.; et al. Zolpidem arousing effect in persistent vegetative state patients: Autonomic, EEG and behavioral assessment. Curr. Pharm. Des. 2014, 20, 4185–4202. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.; et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacino, J.T.; Whyte, J.; Nakase-Richardson, R.; Katz, D.I.; Arciniegas, D.B.; Blum, S.; Day, K.; Greenwald, B.D.; Hammond, F.M.; Pape, T.B.; et al. Minimum competency recommendations for programs that provide rehabilitation services for persons with disorders of consciousness: A position statement of the American Congress of Rehabilitation Medicine and the National Institute on Disability, Independent Living and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch. Phys. Med. Rehabil. 2020, 101, 1072–1089. [Google Scholar] [PubMed]
- Hahm, M.H.; Woo, J. Paradoxical motor and cognitive function recovery in response to zolpidem in a patient with hypoxic-ischemic brain injury. Clin. Psychopharmacol. Neurosci. 2019, 17, 453–457. [Google Scholar] [CrossRef]
- Pignat, J.M.; Mauron, E.; Jöhr, J.; de Keranflec’h, C.G.; Van De Ville, D.; Preti, M.G.; Meskaldji, D.E.; Hömberg, V.; Laureys, S.; Draganski, B.; et al. Outcome prediction of consciousness disorders in the acute stage based on a complementary motor behavioural tool. PLoS ONE 2016, 11, e0156882. [Google Scholar] [CrossRef] [PubMed]
| Full Cohort (n = 146) | Stroke (n = 11) | TBI (n = 87) | ABI * (n = 48) | ||
|---|---|---|---|---|---|
| Age at the time of injury (years; mean ± SD) | 36 ± 15 | 44 ± 13 | 31 ± 14 | 41 ± 16 | |
| Sex (male; female) | 108; 38 | 6; 5 | 69; 18 | 33; 15 | |
| Days since injury on admission (median (IQR)) | 62 (22–246) | 78 (38–119) | 60 (36–119) | 73 (41–180) | |
| Diagnosis on admission | UWS/VS | 63 | 2 | 33 | 28 |
| MCS | 74 | 7 | 48 | 19 | |
| eMCS | 9 | 2 | 6 | 1 | |
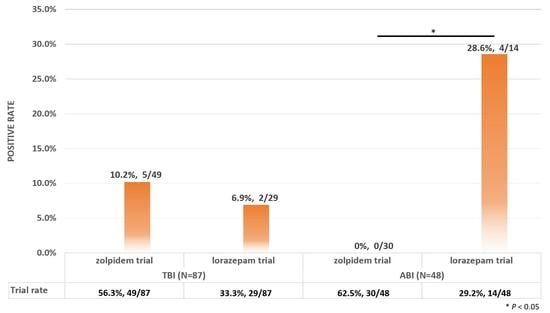
| TBI (n = 87) | ABI (n = 48) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zolpidem Trial | Lorazepam Trial | Zolpidem Trial | Lorazepam Trial | ||||||
| Trial rate * | 56.3% (49/87) | 33.3% (29/87) | 62.5% (30/48) | 29.2% (14/48) | |||||
| Positive rate ** | 10.2% (5/49) | 6.9% (2/29) | 0.0% (0/30) | 28.6% (4/14) | |||||
| R (n = 5) | NR (n = 44) | R (n = 2) | NR (n = 27) | R (n = 0) | NR (n = 30) | R (n = 4) | NR (n = 10) | ||
| Age at the time of injury (years; mean ± SD) | 26 ± 12 | 34 ± 14 | 22 ± 3 | 33 ± 14 | / | 41 ± 15 | 37 ± 11 | 48 ± 14 | |
| Sex (male; female) | 5; 0 | 35; 9 | 2; 0 | 24; 3 | / | 23; 7 | 3; 1 | 7; 3 | |
| Days since injury on admission (median (IQR)) | 62 (32–81) | 64 (45–177) | 42 and 113 | 76 (49–266) | / | 88 (50–202) | 48 (31–78) | 55 (39–106) | |
| Diagnosis on admission | UWS/VS | 2 | 22 | 0 | 9 | / | 20 10 | 1 3 | 5 5 |
| MCS | 3 | 22 | 2 | 18 | |||||
| Case | Sex | Age | Etiology | Trial Time (Days Since Injury) and Regimen | Amantadine | Diagnosis (CRS-R Total (Subscales *)) | Lorazepam Trial | |
|---|---|---|---|---|---|---|---|---|
| Before Trial | After Trial | |||||||
| 1 | Male | 28 | TBI | D56-D60, 10 mg QD D61-D72, 10 mg BID D73-D80, 10 mg TID D81-D82, 10 mg QD D83-D90, 10 mg BID D91-D99, 15 mg BID | D49-D55, 150 mg BID D56-D60, 150 mg BID D61-D72, 150 mg BID D73-D80, 150 mg BID D81-D82, 150 mg BID D83-D89, 150 mg BID D90-D99, 50 mg BID | MCS D42, 7 (004102) D49, 8 (004112) | eMCS D56, 11 (004133) D63, 15 (106233) D70, 11 (006113) D77, 23 (256433) D82, 15 (134133) D91, 15 (105432) D99, 22 (255433) <no CRS-R was administered further> | No |
Behavioral changes/functional improvements: Verbalization.
| ||||||||
| 2 | Male | 18 | TBI | D600, 5 mg ONCE (equivocal) D606-D607, 10 mg QD D611-D617, 10 mg QD D618-D633, 5 mg QD | D593-D599, 100 mg BID D600-D606, 100 mg BID D607-D633, 150 mg BID | MCS D586, 15 (152322) D591, 13 (042322) | eMCS D607, patient was considered clinically eMCS with functional communication. <no CRS-R was administered further> | No |
Behavioral changes/functional improvements: More accurate on IQBA at the evaluation conducted 45 min after taking zolpidem.
| ||||||||
| 3 | Male | 16 | TBI | D46, 5 mg ONCE D47, 7.5 mg ONCE D48-D51, 5 mg QD D52-D136, 5 mg BID | D39-D45, 150 mg BID D46-D47, 150 mg BID D48-D83, 150 mg BID D84-D136, 200 mg BID | eMCS D30, 9 (032112) D32, 10 (032311) D37, 13 (232312) D40, 18 (246312) | eMCS <no CRS-R was administered further> | Equivocal D56 1 mg D58 1 mg |
Behavioral changes/functional improvements: Increased arousal, attention, processing speed.
| ||||||||
| 4 | Male | 46 | TBI | D144, 5 mg QD (no reports regarding responses) D151-D158, 10 mg QD | D137-D142, 100 mg QD D143-D147, 100 mg BID D148-D158, 200 mg BID | UWS/VS D134, 5 (002111) D140, 5 (002111) | UWS/VS D151, 7 (001321) (D158, transferred due to medical deterioration) | No |
Behavioral changes/functional improvements: Reproducible movement to command (during CRS-R).
| ||||||||
| 5 | Male | 23 | TBI | D182, 5 mg ONCE D186, 7.5 mg ONCE D187-D195, 7.5 mg BID D196-D207, 10 mg-5 mg BID (higher PM dose made him sleepy) D208-D210, 15 mg-5 mg BID | D175-D181, 100 mg BID D182-D186, 100 mg BID D187-D195, 100 mg BID D196-D207, 100 mg BID D208-D210, 100 mg BID | MCS, but close to eMCS D131, 14 (152321) D138, 16 (152422) D146, 11 (122321) D152, 16 (152422) D165, 17 (152423) | eMCS D187, 21 (256323) <no CRS-R was administered further> | No |
Behavioral changes/functional improvements: More responsive and interactive.
| ||||||||
| Case | Sex | Age | Etiology | Trial Time (Days Since Injury) and Regimen | Amantadine | Diagnosis (CRS-R Total (Subscales *)) | Zolpidem Trial | |
|---|---|---|---|---|---|---|---|---|
| Before Trial | After Trial | |||||||
| 6 | Male | 48 | ABI | D217, 1 mg ONCE D223, 2 mg ONCE D224-D229, 2 mg TID D230-D231, 2.5 mg TID D232-D233, 3 mg TID D234-D241, 4 mg TID | D210-D216, 100 mg BID D217-D241, 100 mg BID * weaning off of clonazepam (for seizure and spasticity) during the process, discontinued on D238 | MCS D214, 9 (003231) | MCS D223, 12 (142122) D229, 11 (113231) D237, 12 (033231) <no CRS-R was administered further> | Negative D86 10 mg D219 10 mg |
Behavioral changes/functional improvements: Increased arousal and verbalization.
| ||||||||
| 7 | Male | 22 | ABI | D47, 1 mg ONCE D48, 1 mg ONCE D77-D85, 2 mg QD | D40-D46, 100 mg BID D47-D85, 100 mg BID | MCS/eMCS D29, 6 (012111) D33, 8 (021221) D36, 8 (031211) D41, 15 (046212) D44, 8 (032111) | eMCS D50, 14 (135311) D54, 13 (142312) D58, 17 (146222) <no CRS-R was administered further> | Equivocal D54 5 mg D58 10 mg D73 10 mg |
Behavioral changes/functional improvements: Increased cognitive ability.
| ||||||||
| 8 | Male | 24 | TBI | D76, 1 mg ONCE D79, 2 mg ONCE D86-D87, 0.5 mg QD | None | MCS D51, 11 (035111) D58, 12 (035112) D66, 11 (035111) D69, 14 (045122) D73, 11 (035111) | eMCS D79, 14 (035222) D87, 14 (045122) (demonstrated functional object use with both a cup and a pen, however, his attention limits his performance of these behaviors on 4/4 trials) <Patient was considered clinically eMCS; no CRS-R was administered further> | No |
Behavioral changes/functional improvements: Less restless; more attentive and engaged with environment.
| ||||||||
| 9 | Female | 36 | ABI | D96, 1 mg ONCE D97, 2 mg ONCE D99, 1 mg TID D100-D103, 1 mg QD D104-D112, 2 mg QOD D113-D116, 1 mg QD D117-D123 held for washout D124-D130, 1 mg TID D131-D132, 1.5 mg TID D133-D137, 0.5 mg TID D138-D140, 1 mg TID D141-D143, 1 mg QID D144-D145, 1.5 mg QID D146-D149, 2 mg QID D150-D160, 2.5 mg QID | D89-D95, 100 mg BID D96-D109, 100 mg BID Discontinued on D110 | eMCS D33, 6 (012111) D38, 13 (015322) D48, 18 (156321) D52, 18 (155322) D55, 20 (156323) D63, 12 (042321) D66, 17 (145322) (Patient was able to functionally communicate but is often distracted and inattentive.) | eMCS <no CRS-R was administered further> | No |
Behavioral changes/functional improvements: Increased initiation, attention, verbalization, processing time; positive affect; had her best participation in therapy.
| ||||||||
| 10 | Male | 20 | TBI | D296-D300, 1 mg QD D301-D314, 2 mg QD D315-D321, 2 mg-1 mg BID D322-D347, 2 mg BID D348-D379, 1 mg BID | Re-admitted on D295 on 100 mg BID D296-D339, 100 mg BID D340-D379, 150 mg BID | eMCS D147, 15 (045321) D152, 14 (035321) D155, 18 (155421) (Patient demonstrated eMCS by answering >10 egocentric yes/no questions accurately once test was completed.) D158, 13 (035311) D161, 16 (145321) D165, 17 (236321) | eMCS <no CRS-R was administered further> | Negative D133 5 mg D137 10 mg D154 5 mg D349 5 mg |
| ||||||||
| 11 | Male | 41 | TBI&ABI | D136, 1 mg ONCE (negative) D321, 1 mg ONCE D323–324, 1 mg QD D325-D327, 2 mg QD D331-D337, 1 mg QD D338, 2 mg QD D339, 2 mg BID + 2 mg ONCE D340, 3 mg BID D341, 4 mg BID D342-D359, 3 mg BID D390, 2 mg ONCE D393-D394, 3 mg QD D395-D401, 3 mg BID D402-D420, 2 mg BID D421-D430, 2 mg-1 mg BID D431-D433, 1.5 mg-1 mg BID D434-D449, 2 mg-1 mg BID | D314-D320, 200 mg BID D321-D359, 200 mg BID D390-D449, 200 mg BID (D360: Transferred out for scheduled surgery; while at OSH, lorazepam was tapered to stop by Neurology, stating “lorazepam is a CNS depressant and a stimulant effect is pathophysiologically unlikely.”) | eMCS D124, 12 (142311) D128, 13 (151312) D131, 16 (252412) D134, 16 (252412) D137, 16 (252412) D141, 15 (152322) | eMCS <no CRS-R was administered further> | Equivocal D131 5 mgD133 10 mg |
Behavioral changes/functional improvements: Increased alertness, spontaneous movement, and affect.
| ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; O’Brien, K.; Won, W.; Li, S. A Retrospective Analysis on Clinical Practice-Based Approaches Using Zolpidem and Lorazepam in Disorders of Consciousness. Brain Sci. 2021, 11, 726. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11060726
Zhang B, O’Brien K, Won W, Li S. A Retrospective Analysis on Clinical Practice-Based Approaches Using Zolpidem and Lorazepam in Disorders of Consciousness. Brain Sciences. 2021; 11(6):726. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11060726
Chicago/Turabian StyleZhang, Bei, Katherine O’Brien, William Won, and Sheng Li. 2021. "A Retrospective Analysis on Clinical Practice-Based Approaches Using Zolpidem and Lorazepam in Disorders of Consciousness" Brain Sciences 11, no. 6: 726. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11060726