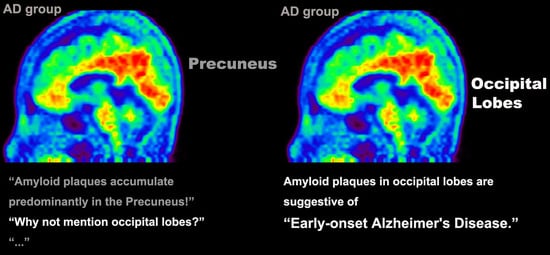
Clinical Implications of Amyloid-Beta Accumulation in Occipital Lobes in Alzheimer’s Continuum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Approval
2.3. Demographics and Clinical Characteristics
2.4. MRI Acquisition and Cortical Thickness Analysis
2.5. [18F]-Florbetaben Amyloid PET Acquisition and Analysis
2.6. [18F]-Florbetaben Amyloid PET Visual Assessment
2.7. Quantitative [18F]-Florbetaben Amyloid PET Analysis
2.8. Neuropsychological Test
2.9. Visuoperceptual Function Tests
2.10. Statistical Analysis
3. Results
3.1. Differences in Clinical Features between OCC+ and OCC- Groups
3.2. Differences in Neuroimaging Findings between OCC+ and OCC− Groups
3.3. Differences in Neuropsychological Tests between OCC+ and OCC− Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dementia 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Barthel, H.; Gertz, H.J.; Dresel, S.; Peters, O.; Bartenstein, P.; Buerger, K.; Hiemeyer, F.; Wittemer-Rump, S.M.; Seibyl, J.; Reininger, C.; et al. Cerebral amyloid-beta pet with florbetaben (18f) in patients with alzheimer’s disease and healthy controls: A multicentre phase 2 diagnostic study. Lancet Neurol. 2011, 10, 424–435. [Google Scholar] [CrossRef]
- Bailly, M.; Ribeiro, M.J.; Vercouillie, J.; Hommet, C.; Gissot, V.; Camus, V.; Guilloteau, D. 18f-fdg and 18f-florbetapir pet in clinical practice: Regional analysis in mild cognitive impairment and alzheimer disease. Clin. Nucl. Med. 2015, 40, e111–e116. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergstrom, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in alzheimer’s disease with pittsburgh compound-b. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Lowe, V.J.; Senjem, M.L.; Weigand, S.D.; Kemp, B.J.; Shiung, M.M.; Knopman, D.S.; Boeve, B.F.; Klunk, W.E.; Mathis, C.A.; et al. 11c pib and structural mri provide complementary information in imaging of alzheimer’s disease and amnestic mild cognitive impairment. Brain A J. Neurol. 2008, 131, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKee, A.C.; Au, R.; Cabral, H.J.; Kowall, N.W.; Seshadri, S.; Kubilus, C.A.; Drake, J.; Wolf, P.A. Visual association pathology in preclinical alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Pikkarainen, M.; Kauppinen, T.; Alafuzoff, I. Hyperphosphorylated tau in the occipital cortex in aged nondemented subjects. J. Neuropathol. Exp. Neurol. 2009, 68, 653–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Crutch, S.J.; Schott, J.M.; Rabinovici, G.D.; Murray, M.; Snowden, J.S.; van der Flier, W.M.; Dickerson, B.C.; Vandenberghe, R.; Ahmed, S.; Bak, T.H.; et al. Consensus classification of posterior cortical atrophy. Alzheimer’s Dementia. 2017, 13, 870–884. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, C.M.; Jeon, S.; Lee, J.M.; Hong, Y.J.; Roh, J.H.; Lee, J.H.; Koh, J.Y.; Na, D.L.; Alzheimer’s Disease Neuroimaging Initiative. Prediction of alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimers Dement. 2016, 2, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Becker, G.A.; Ichise, M.; Barthel, H.; Luthardt, J.; Patt, M.; Seese, A.; Schultze-Mosgau, M.; Rohde, B.; Gertz, H.J.; Reininger, C.; et al. Pet quantification of 18f-florbetaben binding to beta-amyloid deposits in human brains. J. Nucl. Med. 2013, 54, 723–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, B.C.; Bakkour, A.; Salat, D.H.; Feczko, E.; Pacheco, J.; Greve, D.N.; Grodstein, F.; Wright, C.I.; Blacker, D.; Rosas, H.D.; et al. The cortical signature of alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild ad dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 2009, 19, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, D.; Seibyl, J.; Sabbagh, M.; Lai, F.; Hopkins, W.; Bullich, S.; Gimenez, M.; Reininger, C.; Putz, B.; Stephens, A.; et al. Age dependence of brain beta-amyloid deposition in down syndrome: An [18f]florbetaben pet study. Neurology 2015, 84, 500–507. [Google Scholar] [CrossRef]
- Ahn, H.J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fastenau, P.S.; Denburg, N.L.; Hufford, B.J. Adult norms for the rey-osterrieth complex figure test and for supplemental recognition and matching trials from the extended complex figure test. Clin. Neuropsychol. 1999, 13, 30–47. [Google Scholar] [CrossRef]
- Woodard, J.L.; Benedict, R.H.; Roberts, V.J.; Goldstein, F.C.; Kinner, K.M.; Capruso, D.X.; Clark, A.N. Short-form alternatives to the judgment of line orientation test. J. Clin. Exp. Neuropsychol. 1996, 18, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Seo, S.W.; Kim, J.H.; Suh, M.K.; Lee, J.H.; Choe, Y.S.; Lee, K.H.; Kim, J.S.; Kim, G.H.; Noh, Y.; et al. Amyloid deposition in early onset versus late onset alzheimer’s disease. J. Alzheimers Dis. JAD 2013, 35, 813–821. [Google Scholar] [CrossRef]
- Becker, J.T.; Lopez, O.L.; Boller, F. Understanding impaired analysis of faces by patients with probable alzheimer’s disease. Cortex 1995, 31, 129–137. [Google Scholar] [CrossRef]
- Ridgway, G.R.; Lehmann, M.; Barnes, J.; Rohrer, J.D.; Warren, J.D.; Crutch, S.J.; Fox, N.C. Early-onset alzheimer disease clinical variants: Multivariate analyses of cortical thickness. Neurology 2012, 79, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Van der Flier, W.M.; Pijnenburg, Y.A.; Fox, N.C.; Scheltens, P. Early-onset versus late-onset alzheimer’s disease: The case of the missing apoe varepsilon4 allele. Lancet Neurol. 2011, 10, 280–288. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Zwan, M.D.; Tolboom, N.; van Assema, D.M.; Adriaanse, S.F.; Kloet, R.W.; Boellaard, R.; Windhorst, A.D.; Barkhof, F.; Lammertsma, A.A.; et al. Amyloid burden and metabolic function in early-onset alzheimer’s disease: Parietal lobe involvement. Brain A J. Neurol. 2012, 135, 2115–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, G.S.; Gordon, B.A.; Jackson, K.; Christensen, J.J.; Rosana Ponisio, M.; Su, Y.; Ances, B.M.; Benzinger, T.L.S.; Morris, J.C. Tau-pet binding distinguishes patients with early-stage posterior cortical atrophy from amnestic alzheimer disease dementia. Alzheimer Dis. Assoc. Disord. 2017, 31, 87–93. [Google Scholar] [CrossRef]
- Lehmann, M.; Ghosh, P.M.; Madison, C.; Laforce, R., Jr.; Corbetta-Rastelli, C.; Weiner, M.W.; Greicius, M.D.; Seeley, W.W.; Gorno-Tempini, M.L.; Rosen, H.J. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013, 136, 844–858. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.D.; Josephs, K.A.; Machulda, M.M.; Drubach, D.A.; Apostolova, L.G.; Lowe, V.J.; Whitwell, J.L. Clinical, fdg and amyloid pet imaging in posterior cortical atrophy. J. Neurol. 2015, 262, 1483–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintini, I.; Martin, P.R.; Graff-Radford, J.; Senjem, M.L.; Schwarz, C.G.; Machulda, M.M.; Spychalla, A.J.; Drubach, D.A.; Knopman, D.S.; Petersen, R.C.; et al. Longitudinal tau-pet uptake and atrophy in atypical alzheimer’s disease. Neuroimage Clin. 2019, 23, 101823. [Google Scholar] [CrossRef]
- Sintini, I.; Graff-Radford, J.; Senjem, M.L.; Schwarz, C.G.; Machulda, M.M.; Martin, P.R.; Jones, D.T.; Boeve, B.F.; Knopman, D.S.; Kantarci, K.; et al. Longitudinal neuroimaging biomarkers differ across alzheimer’s disease phenotypes. Brain 2020, 143, 2281–2294. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Graff-Radford, J.; Tosakulwong, N.; Weigand, S.D.; Machulda, M.M.; Senjem, M.L.; Spychalla, A.J.; Vemuri, P.; Jones, D.T.; Drubach, D.A.; et al. Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical alzheimer’s disease. Alzheimers Dement. 2018, 14, 1005–1014. [Google Scholar] [CrossRef] [PubMed]

| OCC+ (n = 41) | OCC− (n = 33) | p | |
|---|---|---|---|
| Age (year) | 68.32 ± 10.78 | 74.52 ± 6.52 | 0.003 |
| Gender (Female) | 31 (75.6%) | 20 (60.6%) | 0.166 |
| Education (year) | 10.37 ± 4.10 | 8.76 ± 4.91 | 0.129 |
| Age at Onset (year) | 65.49 ± 11.11 | 72.06 ± 7.09 | 0.003 |
| Disease Duration (year) | 3.22 ± 2.17 | 2.80 ± 1.90 | 0.389 |
| HT | 16 (39.0%) | 23 (69.7%) | 0.009 |
| DM | 3 (7.3%) | 9 (27.3%) | 0.021 |
| Hyperlipidemia | 17 (41.5%) | 13 (39.4%) | 0.857 |
| Disease State | 0.388 | ||
| Alzheimer’s disease | 24 (58.5%) | 16 (48.5%) | |
| Amnestic MCI | 17 (41.5%) | 17 (51.5%) | |
| APOE E4 * | 0.100 | ||
| E4 carrier | 16 (45.7%) | 18 (66.7%) | |
| E4 non-carrier | 19 (54.3%) | 9 (33.3%) | |
| MMSE | 20.46 ± 4.60 | 21.30 ± 4.64 | 0.440 |
| CDR | 0.070 | ||
| 0.5 | 20 (48.8%) | 23 (69.7%) | |
| 1 | 21 (51.2%) | 10 (30.3%) | |
| CDR-SB | 3.94 ± 1.88 | 3.18 ± 1.90 | 0.091 |
| GDS | 4.10 ± 0.80 | 3.67 ± 0.74 | 0.020 |
| GDepS | 13.97 ± 8.19 | 12.28 ± 7.41 | 0.369 |
| Regional SUVR | |||
| Occipital, Left | 1.73 ± 0.20 | 1.49 ± 0.13 | <0.001 |
| Occipital, Right | 1.75 ± 0.19 | 1.49 ± 0.15 | <0.001 |
| Global SUVR | 1.73 ± 0.20 | 1.61 ± 0.16 | 0.005 |
| Aβ Accumulation in the Occipital Lobes Compared to Other Brain Regions | ||||||
| OCC+ (n = 41) | OCC− (n = 32) | Total (n = 73) | ||||
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | |
| Occipital | 1.74 ± 0.19 | 1.49 ± 0.13 | 1.63 ± 0.21 | |||
| Parietal | 1.85 ± 0.23 | <0.001 | 1.70 ± 0.16 | <0.001 | 1.79 ± 0.21 | <0.001 |
| Temporal | 1.61 ± 0.20 | <0.001 | 1.48 ± 0.15 | 0.639 | 1.55 ± 0.19 | <0.001 |
| Frontal | 1.76 ± 0.22 | 0.535 | 1.66 ± 0.21 | <0.001 | 1.71 ± 0.22 | <0.001 |
| Medial frontal | 1.78 ± 0.22 | 0.187 | 1.68 ± 0.21 | <0.001 | 1.73 ± 0.22 | <0.001 |
| PCC | 1.98 ± 0.26 | <0.001 | 1.87 ± 0.25 | <0.001 | 1.93 ± 0.26 | <0.001 |
| Precuneus | 2.03 ± 0.26 | <0.001 | 1.89 ± 0.22 | <0.001 | 1.97 ± 0.25 | <0.001 |
| Central | 1.56 ± 0.18 | <0.001 | 1.46 ± 0.13 | 0.052 | 1.52 ± 0.17 | <0.001 |
| Hippocampus and amygdala | 1.23 ± 0.11 | <0.001 | 1.21 ± 0.08 | <0.001 | 1.22 ± 0.10 | <0.001 |
| Aβ Accumulation between the OCC+ and OCC− Group | ||||||
| OCC+ (n = 41) | OCC− (n = 32) | p | Adjusted p * | |||
| Occipital, Left | 1.73 ± 0.20 | 1.49 ± 0.13 | <0.001 | <0.001 | ||
| Occipital, Right | 1.75 ± 0.19 | 1.49 ± 0.15 | <0.001 | <0.001 | ||
| Parietal, Left | 1.84 ± 0.24 | 1.71 ± 0.16 | 0.006 | 0.872 | ||
| Parietal, Right | 1.85 ± 0.22 | 1.70 ± 0.18 | 0.002 | 0.286 | ||
| Temporal, Left | 1.60 ± 0.21 | 1.49 ± 0.16 | 0.018 | 0.319 | ||
| Temporal, Right | 1.61 ± 0.19 | 1.48 ± 0.16 | 0.002 | 0.220 | ||
| Frontal, Left | 1.75 ± 0.23 | 1.66 ± 0.20 | 0.078 | 0.010 | ||
| Frontal, Right | 1.76 ± 0.21 | 1.65 ± 0.23 | 0.038 | 0.195 | ||
| Medial frontal, Left | 1.77 ± 0.23 | 1.68 ± 0.21 | 0.094 | 0.010 | ||
| Medial frontal, Right | 1.78 ± 0.22 | 1.67 ± 0.23 | 0.043 | 0.081 | ||
| PCC, Left | 1.97 ± 0.26 | 1.87 ± 0.27 | 0.099 | 0.616 | ||
| PCC, Right | 1.99 ± 0.26 | 1.88 ± 0.24 | 0.073 | 0.379 | ||
| Precuneus, Left | 2.04 ± 0.25 | 1.88 ± 0.25 | 0.011 | 0.417 | ||
| Precuneus, Right | 2.02 ± 0.28 | 1.89 ± 0.21 | 0.039 | 0.799 | ||
| Central, Left | 1.56 ± 0.19 | 1.46 ± 0.13 | 0.011 | 0.570 | ||
| Central, Right | 1.57 ± 0.18 | 1.46 ± 0.15 | 0.005 | 0.275 | ||
| Hippocampus and amygdala, Left | 1.23 ± 0.11 | 1.22 ± 0.08 | 0.457 | 0.258 | ||
| Hippocampus and amygdala, Right | 1.24 ± 0.10 | 1.21 ± 0.09 | 0.192 | 0.427 | ||
| OCC+ (n = 41) | OCC− (n = 33) | p | Adjusted p * | Adjusted p † | |
|---|---|---|---|---|---|
| Digit Span Forward | −0.01 ± 1.04 | −0.07 ± 0.98 | 0.795 | 0.681 | 0.843 |
| Digit Span Backward | −0.57 ± 1.42 | −0.42 ± 1.03 | 0.626 | 0.797 | 0.835 |
| K-BNT | −1.71 ± 1.72 | −1.70 ± 1.97 | 0.979 | 0.780 | 0.613 |
| RCFT Copy Score | −4.20 ± 5.19 | −1.04 ± 2.30 | 0.001 | 0.002 | 0.010 |
| SVLT Immediate Recall | −1.62 ± 1.34 | −1.53 ± 1.32 | 0.759 | 0.985 | 0.909 |
| SVLT Delayed Recall | −2.46 ± 1.18 | −1.99 ± 1.22 | 0.097 | 0.138 | 0.129 |
| SVLT Recognition | −2.12 ± 1.78 | −1.85 ± 1.20 | 0.432 | 0.586 | 0.407 |
| RCFT Immediate Recall | −1.98 ± 0.82 | −1.43 ± 0.80 | 0.005 | 0.007 | 0.018 |
| RCFT Delayed Recall | −2.21 ± 0.85 | −1.65 ± 0.83 | 0.006 | 0.009 | 0.014 |
| RCFT Recognition | −2.77 ± 2.39 | −1.38 ± 1.37 | 0.004 | 0.005 | 0.014 |
| COWAT Animal | −1.53 ± 1.13 | −1.14 ± 1.17 | 0.158 | 0.253 | 0.359 |
| COWAT Supermarket | −1.15 ± 1.02 | −0.94 ± 0.88 | 0.374 | 0.591 | 0.713 |
| COWAT Phonemic | −0.86 ± 1.09 | −0.73 ± 1.05 | 0.618 | 0.920 | 0.908 |
| Stroop Color Reading | −2.15 ± 1.82 | −1.60 ± 1.18 | 0.139 | 0.239 | 0.462 |
| OCC+ (n = 12) | OCC− (n = 14) | p | Adjusted p a | Adjusted p b | |
|---|---|---|---|---|---|
| ECFT | 6.25 ± 1.71 | 7.57 ± 2.34 | 0.119 | 0.140 | 0.021 |
| JOLO (Short Form) | 6.50 ± 3.68 | 7.89 ± 3.98 | 0.377 | 0.397 | 0.131 |
| K-FAB | 17.50 ± 2.88 | 17.14 ± 3.39 | 0.777 | 0.867 | 0.082 |
| Color Matching | 9.75 ± 0.87 | 10.00 ± 0.00 | 0.339 | 0.519 | 0.563 |
| Letter Matching | 9.67 ± 0.89 | 9.79 ± 0.58 | 0.685 | 0.899 | 0.463 |
| K-Famous Naming | 20.33 ± 5.19 | 17.39 ± 7.18 | 0.185 | 0.408 | 0.425 |
| Color Naming | 9.36 ± 1.21 | 8.29 ± 2.46 | 0.167 | 0.212 | 0.658 |
| Color Knowledge | 18.46 ± 1.97 | 17.71 ± 2.05 | 0.281 | 0.601 | 0.823 |
| Letter Reading | 9.75 ± 0.87 | 9.86 ± 0.36 | 0.676 | 0.882 | 0.452 |
| Word Reading | 22.75 ± 2.34 | 23.21 ± 2.15 | 0.603 | 0.778 | 0.845 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.; Kim, C.M.; Kim, J.E.; Oh, M.; Oh, J.S.; Yoon, Y.W.; Kim, J.S.; Lee, J.-H.; Roh, J.H. Clinical Implications of Amyloid-Beta Accumulation in Occipital Lobes in Alzheimer’s Continuum. Brain Sci. 2021, 11, 1232. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11091232
Hwang J, Kim CM, Kim JE, Oh M, Oh JS, Yoon YW, Kim JS, Lee J-H, Roh JH. Clinical Implications of Amyloid-Beta Accumulation in Occipital Lobes in Alzheimer’s Continuum. Brain Sciences. 2021; 11(9):1232. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11091232
Chicago/Turabian StyleHwang, Jihye, Chan Mi Kim, Ji Eun Kim, Minyoung Oh, Jungsu S. Oh, Young Wook Yoon, Jae Seung Kim, Jae-Hong Lee, and Jee Hoon Roh. 2021. "Clinical Implications of Amyloid-Beta Accumulation in Occipital Lobes in Alzheimer’s Continuum" Brain Sciences 11, no. 9: 1232. https://0-doi-org.brum.beds.ac.uk/10.3390/brainsci11091232