Cranberry (Vaccinium macrocarpon) Juice Precipitate Pigmentation Is Mainly Polymeric Colors and Has Limited Impact on Soluble Anthocyanin Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Juice Preparation and Accelerated Aging
2.3. Model System Preparation and Accelerated Aging
2.4. Acid Hydrolysis
2.5. Spectrophotometric Analysis Techniques
2.6. HPLC Analysis
2.7. Sample Preparation and Analysis by MALDI-TOF MS
2.8. Statistical Analyses
3. Results and Discussion
3.1. Chemical Composition Cranberry Juice and Model Juice System
3.2. Anthocyanin Loss Rates
3.3. Loss Rate Based on Anthocyanidin
3.4. Loss Rate Based on Glycoside
3.5. Implications of Anthocyanin Loss on Bioactivity of Juice
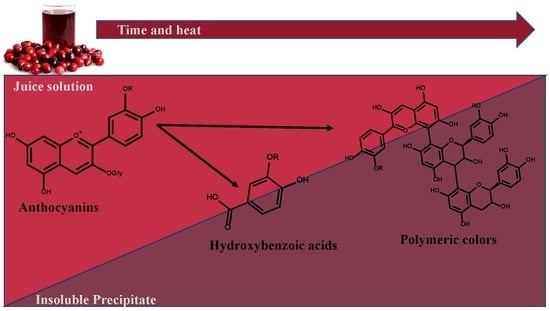
3.6. Formation of Hydroxybenzoic Acids
3.7. Quantitative Summary of Loss of Cyanidin-Glycosides and Peonidin-Glycosides
3.8. Precipitate Analysis
3.9. Polymeric Color Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the health, alcohol and psychosocial factors in Eastern Europe (HAPIEE) study. Br. J. Nutr. 2017, 118, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual intake of dietary flavonoids and risk of parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, A.R.; Varghese, S.; Vita, J.A.; Freedman, J.E. Letters to the editor the antiinflammatory effects of purple. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e179–e180. [Google Scholar]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamport, D.J.; Lawton, C.L.; Merat, N.; Jamson, H.; Myrissa, K.; Hofman, D.; Chadwick, H.K.; Quadt, F.; Wightman, J.D.; Dye, L. Concord grape juice, cognitive function, and driving performance: A 12-Wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am. J. Clin. Nutr. 2016, 103, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Bendokas, V.; Stanys, V.; Mažeikien, I.; Trumbeckaite, S.; Baniene, R.; Liobikas, J. Anthocyanins: From the field to the antioxidants in the body. Antioxidants 2020, 9, 819. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Wightman, J.D. Potential health benefits of (poly)phenols derived from fruit and 100% fruit juice. Nutr. Rev. 2020, 78, 145–174. [Google Scholar] [CrossRef]
- Pannala, A.S.; Rice-Evans, C. Rapid screening method for relative antioxidant activities of flavonoids and phenolics. Methods Enzymol. 2001, 335, 266–272. [Google Scholar]
- Bolling, B.W.; Chen, Y.; Chen, C.O. Contributions of phenolics and added vitamin C to the antioxidant capacity of pomegranate and grape juices: Synergism and antagonism among constituents. Int. J. Food Sci. Technol. 2013, 48, 2650–2658. [Google Scholar] [CrossRef]
- Howard, L.R.; Prior, R.L.; Liyanage, R.; Lay, J.O. Processing and storage effect on berry polyphenols: Challenges and implications for bioactive properties. J. Agric. Food Chem. 2012, 60, 6678–6693. [Google Scholar] [CrossRef] [PubMed]
- Dorris, M.R.; Voss, D.M.; Bollom, M.A.; Krawiec-Thayer, M.P.; Bolling, B.W. Browning index of anthocyanin-rich fruit juice depends on pH and anthocyanin loss more than the gain of soluble polymeric pigments. J. Food Sci. 2018, 83, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mao, Y.; Sui, S.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Hayasaka, Y.; Smith, P.A.; Bindon, K.A. Effect of grape seed and skin tannin molecular mass and composition on the rate of reaction with anthocyanin and subsequent formation of polymeric pigments in the presence of acetaldehyde. J. Agric. Food Chem. 2019, 67, 8938–8949. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal degradation of acylated and nonacylated anthocyanins. J. Food Sci. 2006, 71, C504–C512. [Google Scholar] [CrossRef]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef]
- Sinela, A.; Rawat, N.; Mertz, C.; Achir, N.; Fulcrand, H.; Dornier, M. Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem. 2017, 214, 234–241. [Google Scholar] [CrossRef]
- Sinela, A.M.; Mertz, C.; Achir, N.; Rawat, N.; Vidot, K.; Fulcrand, H.; Dornier, M. Exploration of reaction mechanisms of anthocyanin degradation in a roselle extract through kinetic studies on formulated model media. Food Chem. 2017, 235, 67–75. [Google Scholar] [CrossRef]
- Tanchev, S.; Ioncheva, N. Products of thermal degradation of the anthocyanins cyanidin-3-glucoside, cyanidin-3-rutinoside and cyanidin-3-sophoroside. Nahrung 1976, 20, 889–893. [Google Scholar] [CrossRef]
- Markakis, P. Stability of anthocyanins in foods. In Anthocyanins as Food Colors; Elsevier: Amsterdam, The Netherlands, 1982; pp. 163–180. [Google Scholar]
- Kammerer, D.R. Anthocyanins. In Handbook on Natural Pigments in Food and Beverages; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 61–80. [Google Scholar]
- Markakis, P.; Jurd, L. Anthocyanins and their stability in foods. Crit. Rev. Food Technol. 1974, 4, 437–456. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in Vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the temperature and oxygen exposure in red port wine: A kinetic approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines II. anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Zhang, M.; Tao, G.; Sun, Y.; Sun, J. Chemical composition of clarified bayberry (Myrica rubra Sieb, et Zucc.) juice sediment. J. Agric. Food Chem. 2006, 54, 7710–7716. [Google Scholar] [CrossRef]
- Beveridge, T. Opalescent and cloudy fruit Juices: Formation and particle stability. Crit. Rev. Food Sci. Nutr. 2002, 42, 317–337. [Google Scholar] [CrossRef]
- Zou, B.; Xu, Y.; Wu, J.; Yu, Y.; Xiao, G. Phenolic compounds participating in mulberry juice sediment formation during storage. J. Zhejiang Univ. Sci. B 2017, 18, 854–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millet, M.; Poupard, P.; Le Quéré, J.M.; Bauduin, R.; Guyot, S. Haze in apple-based beverages: Detailed polyphenol, polysaccharide, protein, and mineral compositions. J. Agric. Food Chem. 2017, 65, 6404–6414. [Google Scholar] [CrossRef]
- Siebert, K.J. Haze formation in beverages. LWT-Food Sci. Technol. 2006, 39, 987–994. [Google Scholar] [CrossRef]
- Hagerman, A.E. Fifty years of polyphenol—Protein complexes. In Recent Advances in Polyphenol Research; Cheynier, V., Sarni-Manchado, P., Quideau, S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; Volume 3, pp. 71–97. [Google Scholar]
- Erkan-Koç, B.; Türkyilmaz, M.; Yemiş, O.; Özkan, M. Effects of various protein- and polysaccharide-based clarification agents on antioxidative compounds and colour of pomegranate juice. Food Chem. 2015, 184, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Siebert, K.J. Effects of protein-polyphenol interactions on beverage haze, stabilization, and analysis. J. Agric. Food Chem. 1999, 47, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine—Effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Cerreti, M.; Liburdi, K.; Benucci, I.; Esti, M. The effect of pectinase and protease treatment on turbidity and on haze active molecules in pomegranate juice. LWT-Food Sci. Technol. 2016, 73, 326–333. [Google Scholar] [CrossRef]
- Martin, D.A.; Smyth, J.A.; Liu, Z.; Bolling, B.W. Aronia berry (Aronia mitschurinii ‘Viking’) inhibits colitis in mice and inhibits T Cell tumour necrosis factor-α secretion. J. Funct. Foods 2018, 44, 48–57. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Anthocyanins. In Handbook of Food Analytical Chemistry: Pigments, Colorants, Flavors, Texture, and Bioactive Food Components; Wrolstad, S.J., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., et al., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 5–69. [Google Scholar]
- Ewald, C.; Fjelkner-Modig, S.; Johansson, K.; Sjöholm, I.; Åkesson, B. Effect of processing on major flavonoids in processed onions, green beans, and peas. Food Chem. 1999, 64, 231–235. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Wrolstad, R.E. Color and pigment analyses in fruit products. In Agricultural Experiment Station Oregon State University Station Bulletin; Agricultural Experiment Station, Oregon State University: Corvallis, OR, USA, 1993; Volume 624, pp. 1–17. [Google Scholar]
- U.S. EPA. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2; United States Environmental Protection Agency: Washington, DC, USA, 2016; pp. 1–8. [Google Scholar]
- Krueger, C.G.; Vestling, M.M.; Reed, J.D. Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry of anthocyanin-polyflavan-3-Ol oligomers in cranberry fruit [Vaccinium macrocarpon, Ait.] and spray-dried cranberry juice. In Red Wine Color: Exploring the Mysteries; Waterhouse, A.L., Kennedy, J.A., Eds.; Oxford University Press: Washington, DC, USA, 2004; pp. 232–246. [Google Scholar]
- Tarascou, I.; Mazauric, J.-P.; Meudec, E.; Souquet, J.-M.; Cunningham, D.; Nojeim, S.; Cheynier, V.; Fulcrand, H. Characterisation of genuine and derived cranberry proanthocyanidins by LC-ESI-MS. Food Chem. 2011, 128, 802–810. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen Radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanidins in blueberries and cranberries (Vaccinium Spp.) using high performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Vance, T.M.; Nam, T.G.; Kim, D.O.; Koo, S.I.; Chun, O.K. Evaluation of pH differential and HPLC methods expressed as cyanidin-3-glucoside equivalent for measuring the total anthocyanin contents of berries. J. Food Meas. Charact. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Harnly, J.M.; Doherty, R.F.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Bhagwat, S.; Gebhardt, S. Flavonoid content of U.S. fruits, vegetables, and nuts. J. Agric. Food Chem. 2006, 54, 9966–9977. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States : Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Hong, V.; Wrolstad, R.E. Use of HPLC separation/photodiode array detection for characterization of anthocyanins†. J. Agric. Food Chem. 1990, 38, 708–715. [Google Scholar] [CrossRef]
- Brown, P.N.; Shipley, P.R. Determination of anthocyanins in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2011, 94, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Yamasaki, S.; Mizoguchi, K.; Kodama, N.; Iseki, J. Lowbush blueberry, highbush blueberry and cranberry extracts protect cucumber (Cucumis sativus L.) cotyledons from damage induced by UV-B irradiation. Japan Agric. Res. Q. 2017, 51, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Prior, R.L.; Rogers, T.R.; Khanal, R.C.; Wilkes, S.E.; Wu, X.; Howard, L.R. Urinary excretion of phenolic acids in rats fed cranberry. J. Agric. Food Chem. 2010, 58, 3940–3949. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, C.; Zhan, J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002, 50, 3789–3794. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Roidoung, S.; Dolan, K.D.; Siddiq, M. Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem. 2016, 210, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; Mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Cabrita, L.; Fossen, T.; Andersen, O.M. Colour and stability of the six common anthocyanidin 3-glucosides in aqueous solutions. Food Chem. 2000, 68, 101–107. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Procópio, D.P.; Fontes, E.A.F.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Aging of red wines made from hybrid grape Cv. BRS violeta: Effects of accelerated aging conditions on phenolic composition, color and antioxidant activity. Food Res. Int. 2014, 56, 182–189. [Google Scholar] [CrossRef]
- Trošt, K.; Golc-Wondra, A.; Prošek, M.; Milivojevič, L. Anthocyanin degradation of blueberry-aronia nectar in glass compared with carton during storage. J. Food Sci. 2008, 73, 405–411. [Google Scholar] [CrossRef]
- Attoe, E.L.; von Elbe, J.H. Photochemial degradation of betanine and selected anthocyanins. J. Food Sci. 1981, 46, 1934–1937. [Google Scholar] [CrossRef]
- Farr, J.E.; Sigurdson, G.T.; Giusti, M.M. Stereochemistry and glycosidic linkages of C3-glycosylations affected the reactivity of cyanidin derivatives. Food Chem. 2019, 278, 443–451. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Oikawa, K.; Tateyama, C.; Konishi, T. Acid mediated hydrolysis of blueberry anthocyanins. Chem. Pharm. Bull. 2001, 49, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2014, 62, 4018–4025. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, Y.; Yoon, H.; Park, H.; Song, S.; Yeum, K. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Chemical quality parameters and anthocyanin pattern of red-fleshed weirouge apples. J. Appl. Bot. Food Qual. 2006, 80, 82–87. [Google Scholar]
- Gudžinskaitė, I.; Stackevičienė, E.; Liaudanskas, M.; Zymonė, K.; Žvikas, V.; Viškelis, J.; Urbštaitė, R.; Janulis, V. Variability in the qualitative and quantitative composition and content of phenolic compounds in the fruit of introduced American cranberry (Vaccinium macrocarpon Aiton). Plants 2020, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zuo, Y. Identification of flavonol glycosides in American cranberry fruit. Food Chem. 2007, 101, 1357–1364. [Google Scholar] [CrossRef]
- Chen, H.; Zuo, Y.; Deng, Y. Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chromatogr. A 2001, 913, 387–395. [Google Scholar] [CrossRef]
- Wang, Y.; Johnson-Cicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acids over fruit development in american cranberry (Vaccinium macrocarpon) cultivars using HPLC and APCI-MS/MS. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törronen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Makris, D.P.; Rossiter, J.T. Heat-induced, metal-catalyzed oxidative degradation of quercetin and rutin (Quercetin 3-O-Rhamnosylglucoside) in aqueous model systems. J. Agric. Food Chem. 2000, 48, 3830–3838. [Google Scholar] [CrossRef]
- Kellil, A.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Empirical kinetic modelling and mechanisms of quercetin thermal degradation in aqueous model systems: Effect of pH and addition of antioxidants. Appl. Sci. 2021, 11, 2579. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of Thermal Processing on the Flavonols Rutin and Quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Krueger, C.G.; Shanmuganayagam, D.; Vestling, M.M.; Reed, J.D. Deconvolution of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry isotope patterns to determine ratios of A-type to B-type interflavan bonds in cranberry proanthocyanidins. Food Chem. 2012, 135, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Alvarado, D.; Alfaro-Viquez, E.; Krueger, C.G.; Vestling, M.M.; Reed, J.D. Classification of proanthocyanidin profiles using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) spectra data combined with multivariate analysis. Food Chem. 2021, 336, 127667. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Alvarado, D.; Alfaro-Viquez, E.; Krueger, C.G.; Vestling, M.M.; Reed, J.D. Identification of A-type proanthocyanidins in cranberry-based foods and dietary supplements by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, first action method: 2019.05. J. AOAC Int. 2021, 104, 223–231. [Google Scholar] [CrossRef] [PubMed]


| Analyte | Initial Value b | Pseudo-Reaction Order c | kd | t1/2 (Days) e |
|---|---|---|---|---|
| cranberry juice supernatant | ||||
| gallic acid | 15.9 ± 0.1 | 0th | 6.0 ± 0.1 | 1.32 ± 0.03 |
| protocatechuic acid | 95 ± 2 | 0th | 32.5 ± 0.5 | 1.46 ± 0.04 |
| vanillic acid | 38.6 ± 0.7 | 0th | 2.8 ± 0.2 | 6.9 ± 0.5 |
| cyanidin 3-O-galactoside | 46.45 ± 0.04 | 1st | −0.290 ± 0.002 | −2.39 ± 0.02 |
| cyanidin 3-O-glucoside | 4.29 ± 0.01 | 1st | −0.281 ± 0.002 | −2.47 ± 0.02 |
| cyanidin 3-O-arabinoside | 81.11 ± 0.05 | 1st | −0.359 ± 0.003 | −1.93 ± 0.02 |
| peonidin 3-O-galactoside | 46.02 ± 0.04 | 1st | −0.311 ± 0.003 | −2.23 ± 0.02 |
| peonidin 3-O-glucoside | 10.43 ± 0.01 | 1st | −0.296 ± 0.0003 | −2.34 ± 0.02 |
| peonidin 3-O-arabinoside | 40.47 ± 0.03 | 1st | −0.373 ± 0.003 | −1.86 ± 0.02 |
| anthocyanins (sum) | 228.7 ± 0.1 | 1st | −0.327 ± 0.003 | −2.12 ± 0.02 |
| anthocyanins (pH differential) | 150 ± 7 | 1st | −0.230 ± 0.004 | −3.00 ± 0.05 |
| polymeric color (%) | 20. ± 2% | 0th | 3.5 ± 0.2 | 2.8 ± 0.1 |
| browning index | 2.1 ± 0.2 | 2nd | 0.085 ± 0.002 | 5.7 ± 0.7 |
| isolated cranberry juice anthocyanins | ||||
| gallic acid | 0.7 ± 0.3 | 0th | 0.22 ± 0.02 | 1.5 ± 0.7 |
| protocatechuic acid | 2.0 ± 0.4 | 0th | 3.6 ± 0.3 | 0.27 ± 0.06 |
| vanillic acid | 0.5 ± 0.2 | 0th | 1.4 ± 0.1 | 0.16 ± 0.06 |
| cyanidin 3-O-galactoside | 28 ± 7 | 1st | −0.139 ± 0.007 | −5.0 ± 0.3 |
| cyanidin 3-O-arabinoside | 34 ± 8 | 1st | −0.197 ± 0.009 | −3.5 ± 0.2 |
| peonidin 3-O-galactoside | 28 ± 6 | 1st | −0.15 ± 0.02 | −4.6 ± 0.7 |
| peonidin 3-O-glucoside | 8 ± 2 | 1st | −0.12 ± 0.02 | −6 ± 1 |
| peonidin 3-O-arabinoside | 21 ± 7 | 1st | −0.20 ± 0.02 | −3.4 ± 0.4 |
| anthocyanins (sum) | 120 ± 30 | 1st | −0.159 ± 0.008 | −4.4 ± 0.2 |
| Juice Fraction | Component | Concentration in Fresh Sample (µM) b | Concentration after 10 Days at 50 °C (µM) b | ΔConcentration (µM) | |
|---|---|---|---|---|---|
| whole cranberry juice | |||||
| soluble components | cyanidin glycosides | 131.84 ± 0.08 | 4.3 ± 0.4 c | −127.5 ± 0.4 | 100% |
| protocatechuic acid | 95 ± 2 | 430 ± 20 c | 340 ± 20 | 260 ± 10% e | |
| extract from precipitate | cyanidin glycosides | <12 | <12 | 0 | 0% |
| protocatechuic acid | 0.5 ± 0.6 | 10 ± 10 c | 12 ± 9 | 9 ± 7% | |
| hydrolysate of precipitate | cyanidin | <12 | 90 ± 30 c | 90 ± 30 | 70 ± 20% e |
| protocatechuic acid | <0.46 | 4 ± 1 c | 4 ± 1 | 3.2 ± 0.9% | |
| soluble components | peonidin glycosides | 96.91 ± 0.07 | 3.0 ± 0.3 d | −93.9 ± 0.3 | 100% |
| vanillic acid | 38.6 ± 0.7 | 67 ± 2 d | 29 ± 2 | 31 ± 2% | |
| extract from precipitate | peonidin glycosides | <0.50 | <0.50 | 0 | 0% |
| vanillic acid | <0.27 | 2 ± 1 d | 2 ± 1 | 2 ± 1% | |
| hydrolysate of precipitate | peonidin | <0.51 | 1.1 ± 0.3 c | 1.0 ± 0.3 | 1.1 ± 0.4% |
| vanillic acid | <0.27 | <0.27 | 0 | 0% | |
| isolated cranberry juice anthocyanins | |||||
| soluble components | cyanidin glycosides | 70 ± 20 | 11 ± 2 c | −50 ± 20 | 100% |
| protocatechuic acid | 2.0 ± 0.4 | 35 ± 3 c | 33 ± 3 | 70 ± 20% e | |
| extract from precipitate | cyanidin glycosides | <11 | <11 | 0 | 0% |
| protocatechuic acid | <0.39 | 0.6 ± 0.5 | 0.6 ± 0.5 | 1.0 ± 0.5% | |
| hydrolysate of precipitate | cyanidin | <1.3 | 6 ± 5 | 6 ± 5 | 9 ± 6% e |
| protocatechuic acid | <2.6 | 3.6 ± 0.5 c | 3.1 ± 0.5 | 6 ± 1% | |
| soluble components | peonidin glycosides | 50 ± 20 | 11.8 ± 0.8 c | −40 ± 20 | 100% |
| vanillic acid | 0.5 ± 0.2 | 15 ± 3 c | 14 ± 3 | 35 ± 5% | |
| extract from precipitate | peonidin glycosides | <12 | <12 | 0 | 0% |
| vanillic acid | <0.33 | <0.33 | 0 | 0% | |
| hydrolysate of precipitate | peonidin | <0.70 | 0.8 ± 0.6 | 0.7 ± 0.6 | 1.6 ± 0.8% |
| vanillic acid | <0.68 | <0.68 | 0 | 0% | |
| Juice Fraction | Sephadex Fraction b | Anthocyanin | # (Epi)catechin Units | # A-Type Linkages | Anthocyanin-Procyanidin Connection |
|---|---|---|---|---|---|
| unaged juice | |||||
| whole juice | alcohols | cyanidin-hexoside | 1–4 | 0–2 | ethylene cross-linked, directly bonded |
| cyanidin-pentoside | 1–3 | 0–1 | ethylene cross-linked | ||
| peonidin-hexoside | 1–4 | 0–2 | ethylene cross-linked, directly bonded | ||
| peonidin-pentoside | 1 and 3 | 0–1 | ethylene cross-linked | ||
| acetone/water | cyanidin-hexoside | 2 | 0 | ethylene cross-linked | |
| cyanidin-pentoside | 1 | 0 | ethylene cross-linked | ||
| peonidin-hexoside | 1–2 | 0–1 | directly bonded | ||
| peonidin-pentoside | 2 | 0–1 | ethylene cross-linked | ||
| aged juice | |||||
| supernatant c | alcohols | cyanidin-hexoside | 2–3 | 1–2 | ethylene cross-linked |
| cyanidin-pentoside | 2 | 1 | ethylene cross-linked | ||
| peonidin-hexoside | 2–3 | 1–2 | ethylene cross-linked, directly bonded | ||
| peonidin-pentoside | 2 | 0–1 | ethylene cross-linked | ||
| acetone/water | cyanidin-hexoside | 2 | 0 | ethylene cross-linked | |
| cyanidin-pentoside | 1 | 0 | ethylene cross-linked | ||
| peonidin-hexoside | 2 | 0–1 | ethylene cross-linked, directly bonded | ||
| peonidin-pentoside | 2 | 0–1 | ethylene cross-linked, | ||
| precipitate c | alcohols | cyanidin-hexoside | 2 | 0 | ethylene cross-linked |
| peonidin-hexoside | 2 | 1 | directly bonded | ||
| peonidin-pentoside | 2 | 0–1 | ethylene cross-linked | ||
| acetone/water | cyanidin-hexoside | 2 | 1 | ethylene cross-linked | |
| cyanidin-pentoside | 1 | 0 | ethylene cross-linked | ||
| peonidin-hexoside | 2 | 1 | directly bonded | ||
| peonidin-pentoside | 2 | 0–1 | ethylene cross-linked | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorris, M.R.; Bolling, B.W. Cranberry (Vaccinium macrocarpon) Juice Precipitate Pigmentation Is Mainly Polymeric Colors and Has Limited Impact on Soluble Anthocyanin Loss. Antioxidants 2021, 10, 1788. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10111788
Dorris MR, Bolling BW. Cranberry (Vaccinium macrocarpon) Juice Precipitate Pigmentation Is Mainly Polymeric Colors and Has Limited Impact on Soluble Anthocyanin Loss. Antioxidants. 2021; 10(11):1788. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10111788
Chicago/Turabian StyleDorris, Matthew R., and Bradley W. Bolling. 2021. "Cranberry (Vaccinium macrocarpon) Juice Precipitate Pigmentation Is Mainly Polymeric Colors and Has Limited Impact on Soluble Anthocyanin Loss" Antioxidants 10, no. 11: 1788. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10111788