Peroxiredoxin 6 Applied after Exposure Attenuates Damaging Effects of X-ray Radiation in 3T3 Mouse Fibroblasts
Abstract
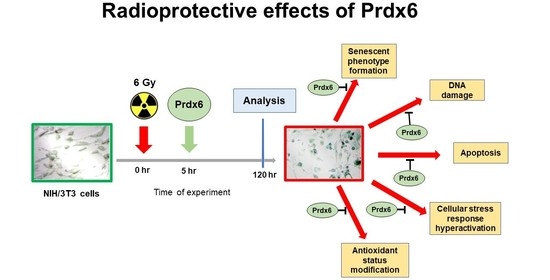
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Evaluation of Cell Proliferation
2.2. X-ray Treatment
2.3. Isolation and Purification of the PRDX6
2.4. Senescence-Associated Beta-Galactosidase Staining
2.5. Electrophoresis and Immunoblotting
2.6. Gene Expression Analysis
2.7. Measurement of Cytokine Production
2.8. Statistical Analysis
3. Results
3.1. Effects of Prdx6 on the Survival, Proliferation, and Antioxidant Status of Irradiated 3T3 Cells
3.2. Post-Irradiation Effects of Prdx6 on Cytokine Production, TLR’s Expression, Apoptosis, and Cellular Stress in Irradiated 3T3 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively generated base damage to cellular DNA. Free Radic. Biol. Med. 2010, 49, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, A.E.; Sharapov, M.G.; Tikhonova, I.V.; Chemeris, N.K.; Fesenko, E.E.; Novoselov, V.I.; Temnov, A.A. Vascular pathology of ischemia/reperfusion injury of rat small intestine. Cells Tissues Organs 2017, 203, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Palutina, O.A.; Sharapov, M.G.; Temnov, A.A.; Novoselov, V.I. Nephroprotective Effect Exogenous Antioxidant Enzymes during Ischemia/Reperfusion-Induced Damage of Renal Tissue. Bull. Exp. Biol. Med. 2016, 160, 322–326. [Google Scholar] [CrossRef]
- Karaduleva, E.V.; Mubarakshina, E.K.; Sharapov, M.G.; Volkova, A.E.; Pimenov, O.Y.; Ravin, V.K.; Kokoz, Y.M.; Novoselov, V.I. Cardioprotective effect of modified peroxiredoxins in retrograde perfusion of isolated rat heart under conditions of oxidative stress. Bull. Exp. Biol. Med. 2016, 160, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Novoselova, E.G.; Glushkova, O.V.; Parfenuyk, S.B.; Khrenov, M.O.; Lunin, S.M.; Novoselova, T.V.; Sharapov, M.G.; Shaev, I.A.; Novoselov, V.I. Protective effect of peroxiredoxin 6 against toxic effects of glucose and cytokines in pancreatic RIN-m5F βcells. Biochemistry 2019, 84, 637–643. [Google Scholar] [CrossRef]
- Forshaw, T.E.; Holmila, R.; Nelson, K.J.; Lewis, J.E.; Kemp, M.L.; Tsang, A.W.; Poole, L.B.; Lowther, W.T.; Furdui, C.M. Peroxiredoxins in cancer and response to radiation therapies. Antioxidants 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wang, Y.; Su, Y. Peroxiredoxins, a novel target in cancer radiotherapy. Canc. Lett. 2009, 286, 154–160. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Glushkova, O.V.; Parfenyuk, S.B.; Gudkov, S.V.; Lunin, S.M.; Novoselova, E.G. The role of TLR4/NF-κB signaling in the radioprotective effects of exogenous Prdx6. Arch. Biochem. Biophys. 2021, 702, 108830. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Gudkov, S.V.; Gordeeva, A.E.; Karp, O.E.; Ivanov, V.E.; Shelkovskaya, O.V.; Bruskov, V.I.; Novoselov, V.I.; Fesenko, E.E. Peroxiredoxin 6 is a natural radioprotector. Dokl. Biochem. Biophys. 2016, 467, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, M.G.; Novoselov, V.I.; Fesenko, E.E.; Bruskov, V.I.; Gudkov, S.V. The role of peroxiredoxin 6 in neutralization of X-ray mediated oxidative stress: Effects of gene expression, preservation of radiosensitive tissues and postradiation survival of animals. Free Radic. Res. 2017, 51, 148–166. [Google Scholar] [CrossRef]
- Sharapov, M.G.; Novoselov, V.I.; Gudkov, S.V. Radioprotective role of peroxiredoxin 6. Antioxidants 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Sharapov, M.G.; Gudkov, S.V.; Lankin, V.Z.; Novoselov, V.I. Role of Glutathione Peroxidases and Peroxiredoxins in Free Radical-Induced Pathologies. Biochemistry 2021, 86, 1418–1433. [Google Scholar] [CrossRef]
- Turtoi, A.; Brown, I.; Oskamp, D.; Schneeweisset, F.H.A. Early gene expression in human lymphocytes after gamma-irradiation-a genetic pattern with potential for biodosimetry. Int. J. Radiat. Biol. 2008, 84, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Kabacik, S.; Finnon, P.; Bouffler, S.; Badieet, C. High and low dose responses of transcriptional biomarkers in ex vivo X-irradiated human blood. Int. J. Radiat. Biol. 2013, 89, 512–522. [Google Scholar] [CrossRef]
- Turtoi, A.; Brown, I.; Schlager, M.; Schneeweisset, F.H.A. Gene expression profile of human lymphocytes exposed to (211)At alpha particles. Radiat. Res. 2010, 174, 125–136. [Google Scholar] [CrossRef]
- Van de Kamp, G.; Heemskerk, T.; Kanaar, R.; Essers, J. DNA Double Strand Break Repair Pathways in Response to Different Types of Ionizing Radiation. Front. Genet. 2021, 12, 738230. [Google Scholar] [CrossRef]
- Bai, M.; Ma, X.; Li, X.; Wang, X.; Mei, Q.; Li, X.; Wu, Z.; Han, H. The accomplices of NF-κB lead to radioresistance. Review Curr. Protein Pept. Sci. 2015, 16, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.F.; Yan, P.W.; Wang, L.J.; Liu, Y.T.; Wen, J.; Zhang, Q.; Fan, Y.X.; Luo, Y.H. Protective properties of Huperzine A through activation Nrf2/ARE-mediated transcriptional response in X-rays radiation-induced NIH3T3 cells. J. Cel.l Biochem. 2018, 119, 8359–8367. [Google Scholar] [CrossRef]
- Kuang, Y.; Kang, J.; Li, H.; Liu, B.; Zhao, X.; Li, L.; Jin, X.; Li, Q. Multiple functions of p21 in cancer radiotherapy. J. Cancer. Res. Clin. Oncol. 2021, 147, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.M.; El-Kadi, A.O.S. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr. Drug. Metab. 2009, 10, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Zaher, N.H.; Salem, A.A.M.; Ismail, A.F.M. Novel amino acid derivatives bearing thieno[2,3-d]pyrimidine moiety down regulate NF-κB in rats’ liver injury induced by γ-irradiation. J. Photochem. Photobiol. B 2016, 165, 328–339. [Google Scholar] [CrossRef]
- Chen, X.L.; Dodd, G.; Thomas, S.; Zhang, X.; Wasserman, M.A.; Rovin, B.H.; Kunsch, C. Activation of Nrf2/ARE pathway protects endothelial cells from oxidant injury and inhibits inflammatory gene expression. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1862–H1870. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.Z.; Zhao, Y.; Kanchana, K.; Zhang, Y.; Khan, Z.A.; Chakrabarti, S.; Wu, L.; Wang, J.; Liang, G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-kB both in vitro and in vivo. J. Mol. Cell. Cardiol. 2015, 79, 1–12. [Google Scholar] [CrossRef]
- Goncharov, R.G.; Rogov, K.A.; Temnov, A.A.; Novoselov, V.I.; Sharapov, M.G. Protective role of exogenous recombinant peroxiredoxin 6 under ischemia reperfusion injury of kidney. Cell. Tissue Res. 2019, 378, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Eccles, M.; Li, C.G. Senescence associated beta-galactosidase staining. Bio-Protocol 2012, 2, e247. [Google Scholar] [CrossRef]
- Glushkova, O.V.; Khrenov, M.O.; Novoselova, T.V.; Lunin, S.M.; Parfenyuk, S.B.; Alekseev, S.I.; Fesenko, E.E.; Novoselova, E.G. The role of the NF-κB, SAPK/JNK, and TLR4 signalling pathways in the responses of RAW 264.7 cells to extremely low-intensity microwaves. Int. J. Radiat. Biol. 2015, 91, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Glushkova, O.V.; Parfenyuk, S.B.; Novoselova, T.V.; Khrenov, M.O.; Lunin, S.M.; Novoselova, E.G. The role of p38 and CK2 protein kinases in the response of RAW 264.7 macrophages to lipopolysaccharide. Biochemistry 2018, 83, 746–754. [Google Scholar] [CrossRef]
- Liu, Y.; Tavana, O.; Gu, W. P53 modifications: Exquisite decorations of the powerful guardian. J. Mol. Cell Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Christmann, M.; Kaina, B. Transcriptional regulation of human DNA repair genes following genotoxic stress: Trigger mechanisms, inducible responses and genotoxic adaptation. Nucleic Acids Res. 2013, 41, 8403–8420. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Bruskov, V.I.; Karp, O.E.; Garmash, S.A.; Shtarkman, I.N.; Chernikov, A.V.; Gudkov, S.V. Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action. Free Radic. Res. 2012, 46, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A.B. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid. Redox. Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef] [Green Version]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, inflammation and the immune response in cancer. Mammal. Genome 2018, 29, 843–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, D.; Zhang, Y.; Zheng, J. Regulation of p53: A collaboration between Mdm2 and Mdmx. Oncotarget 2012, 3, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T. Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D2 and E2 production in macrophages in acute inflammation. Free Radic. Biol. Med. 2015, 88, 189–198. [Google Scholar] [CrossRef]
- Hellweg, C.E. The Nuclear Factor κB pathway: A link to the immune system in the radiation response. Cancer Lett. 2015, 368, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; St. Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Lei, X.; Li, X.; Cai, J.M.; Gao, F.; Yang, Y.Y. Toll-like receptors and radiation protection. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 31–39. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Georgakilas, A.G.; Martin, O.A.; Bonner, W.M. p21: A two-faced genome guardian trends. Mol. Med. 2017, 23, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, S.; Khodaverdian, S.; Dorri-Giv, M.; Mohammad Hosseini, S.; Souri, S.; Abedi-Firouzjah, R.; Zamani, H.; Dastranj, L.; Farhood, B. The radioprotective effects of alpha-lipoic acid on radiotherapy-induced toxicities: A systematic review. Int. Immunopharm. 2021, 96, 107741. [Google Scholar] [CrossRef]
- El-Gazzar, M.G.; Zaher, N.H.; El-Hossary, E.M.; Ismail, A.F.M. Radio-protective effect of some new curcumin analogues. J. Photochem. Photobiol. B 2016, 162, 694–702. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novoselova, E.G.; Sharapov, M.G.; Lunin, S.M.; Parfenyuk, S.B.; Khrenov, M.O.; Mubarakshina, E.K.; Kuzekova, A.A.; Novoselova, T.V.; Goncharov, R.G.; Glushkova, O.V. Peroxiredoxin 6 Applied after Exposure Attenuates Damaging Effects of X-ray Radiation in 3T3 Mouse Fibroblasts. Antioxidants 2021, 10, 1951. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10121951
Novoselova EG, Sharapov MG, Lunin SM, Parfenyuk SB, Khrenov MO, Mubarakshina EK, Kuzekova AA, Novoselova TV, Goncharov RG, Glushkova OV. Peroxiredoxin 6 Applied after Exposure Attenuates Damaging Effects of X-ray Radiation in 3T3 Mouse Fibroblasts. Antioxidants. 2021; 10(12):1951. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10121951
Chicago/Turabian StyleNovoselova, Elena G., Mars G. Sharapov, Sergey M. Lunin, Svetlana B. Parfenyuk, Maxim O. Khrenov, Elvira K. Mubarakshina, Anna A. Kuzekova, Tatyana V. Novoselova, Ruslan G. Goncharov, and Olga V. Glushkova. 2021. "Peroxiredoxin 6 Applied after Exposure Attenuates Damaging Effects of X-ray Radiation in 3T3 Mouse Fibroblasts" Antioxidants 10, no. 12: 1951. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10121951